Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2024) Volume 40, Issue 3
Identification of elite native rhizobia adapted to local environmental conditions could offer the opportunity to improve symbiotic nitrogen fixation. This study aimed to evaluate the efficiency of endogenous rhizobia strains associated to soybean root in Benin. A total of 102 presumptive rhizobia strains were isolated from root nodules of soybean. Authentication test showed that the highest nodules scores were obtained with the isolates LMSEM 22 (4.8), LMSEM 101 (4.8), LMSEM 97 (3.2), LMSEM 46 (3.1) and LMSEM 25 (2.8). After authentication, 28 isolates and four reference strains (IRAT FA3, STM3043, STM3045 and USDA110) were tested in greenhouse for their symbiotic effectiveness. Results indicated that isolates LMSEM 101 and LMSEM 22 induced the highest nodules number (46 and 30 nodules plant-1 respectively) and the best dry shoot (7.4 g and 7.1 g plant-1) and outperformed all the reference strains. Afterwards, the five best performing isolates (LMSEM 22, LMSEM 25, LMSEM 53, LMSEM 97, LMSEM 101) were evaluated on three different soils (S1, S2 and S3) comparing them to reference strains IRAT FA3 and STM3043 in the greenhouse. The isolates LMSEM 22, LMSEM 101 and LMSEM 97 outperformed the two reference strains on the three soils but the highest values were obtained on soils S1 and S2. These results confirm the presence of effective endogenous rhizobia on soybean root in Benin.
Legumes; Native Rhizobium strains; Symbiotic efficiency; Soil type; Benin
Nitrogen is the most element that significantly limits yields in many agricultural production systems [1]. Intensive farming practices that achieve high yields require chemical fertilizers which are not only costly but may also create environmental problems [2]. Nitrogen can be supplied through Biological Nitrogen Fixation (BNF) by symbiosis with efficient nitrogen fixing bacteria like Rhizobium [3]. Biological nitrogen fixation is an environment friendly and the cheapest alternative in which Rhizobium fix atmospheric nitrogen into soil by interacting with legumes [4]. Indeed, Rhizobium is the general term used to describe soil diazotrophic gram-negative bacteria that can produce N2-fixing nodules on the roots of legumes [5]. In addition to N2 fixation, rhizobia can act as plant growth promoting bacteria via various mechanisms such as phytohormone and siderophore production phosphate solubilization and biocontrol activities [6].
Since the use of rhizobia as a bio fertilizer is a friendly environmental alternative to chemical fertilization, legumes inoculation with efficient rhizobia strains is widely used in several agricultural systems [7,8]. Studies carried out in Benin showed that inoculation with introduced Bradyrhizobium strains improved soybean and groundnut production compared to the control uninoculated [9,10]. Despite the positive response the yields were not optimal. This could be due to the rivalry with native rhizobia strains. Indeed, studies have indicated that as few as ten competitive native rhizobia strains per gram of soil could be an efficient barrier to exogenous strains in some soils [8,11]. An alternative could be the improvement of the efficiency of rhizobia strains by the identification of native rhizobia with high competitive and symbiotic abilities since endogenous rhizobia strain fit one’s surroundings [12]. Several studies showed that screening native Rhizobium strains with competitive and effective abilities is very beneficial for nitrogen fixation [3,13,14]. Over the past few years, selection of efficient and competitive rhizobia has been assessed systematically for common bean, soybean and peanuts and for other legumes [3,5,13]. However, the presence and effectiveness of native rhizobia strains modulating legumes in Benin soils has not been established. Therefore, the objective of the present study was to assess the effectiveness of endogenous rhizobia associated to soybean, a legume with a great economic and nutritional value to improve symbiotic nitrogen fixation in Benin.
Sampling sites and nodules collection
Presumptive rhizobia strains examined were isolated from soybean root nodules sampled during the flowering stage in three Agro-Ecological Zones (AEZ) of Benin. Nodules were collected from twelve fields per AEZ. Locations where nodules were sampled are shown in (Figure 1). Some general characteristics of these eco-regions are summarized in Table 1. An average of 30-50 nodules was collected by field. Nodules were preserved by dehydration in glass vials containing silica gel [15]. The glass bottle is filled to two-thirds of its volume by silica gel and nodules were subsequently placed between two layers of cotton. Location and the sample collection date were written down on each bottle.
Figure 1: Localization of studied fields in the three agro ecological zones of Benin.
| Agro ecological zone | Climate | Annual rainfall | Types of soils | Grown crops |
|---|---|---|---|---|
| AEZ 3 | Sudanese | 900 to 1.300 mm/year | Ferruginous | Yam, maize, cassava, rice, cotton, soybean, groundnut |
| AEZ 4 | Sudanese tending to sahelian | 800 to 1.300 mm/year | Ferruginous with low water supply | Rice, maize, yam, cassava, soybean, groundnut |
| AEZ 5 | Sudano-guinean | 1.100 to 1.400 mm/year | Ferruginous | Yam, maize, cassava, cowpea, rice, cotton, soybean, groundnut |
Table 1: General characteristics of the three Agro Ecological Zones (AEZ) prospected.
Isolation of presumptive rhizobia strains
The nodules were rehydrated in aseptic condition in sterile distilled water for one night. Root nodules were surface sterilized by washing for 30s with 95% ethanol, immersed in 3% sodium hypochlorite and finally were washed six times with sterile distilled water [16]. A single surface-sterilized nodule was placed into Petri dish and crushed with sterile glass rod in sterile distilled water. A platinum handle was used to streak a loopful of the grounded nodule across the surface of Petri dish which contained Yeast Extract Mannitol Agar (YEMA) medium supplemented with 0.025 g-1 congo red and incubated at 30°C for 2 to 7 days. The YEMA medium used was prepared with the following composition (1 gL-1) yeast extract; 10 gL-1 mannitol; 0.5 gL-1 K2HPO4; 0.2 gL-1 MgSO4.7H2O; 0.1 gL-1 NaCl; 15 gL-1 agar-agar; pH=6.8 [15].
Purification and preservation of isolates
Purity of individual colonies was checked by repeating streaking single colonies on YEMA medium. To ensure purity of presumptive Rhizobium strains, gram test was performed according to Vincent [15]. The YEMA medium was enriched with 25 μg /ml of Bromothymol Blue (BTB) to selectively identify slow grower from fast grower [15]. After purification and based on the morphological characteristics of the colonies, each isolate was known by a given code LMSEM (Laboratoire de Microbiologie des Sols et d’Ecologie Microbienne) followed by a number. Afterwards, isolates were preserved in the refrigerator at 4°C for short term storage and frozen at -80°C in 20% glycerol-YEM broth for long term storage.
Authentication of isolates
The nodulation ability of the strains on soybean was performed as described by Vincent [15]. This experiment was carried out at the greenhouse of Soil Microbiology and Microbial Ecology Laboratory of the Faculty of Agronomic Sciences (University of Abomey-Calavi). Soil used as substratum was N-deficient and was sampled from the beach near the Atlantic Ocean in Cotonou [16]. The experimental design was completely randomized, consisting of three replicates of 107 treatments (102 isolates, 04 reference strains and a non-inoculated used as control). The reference strains used in this study were obtained from Laboratory of Mediterranean and Tropical Symbiosis of Montpellier (strains IRAT FA3, STM3043 and STM3045) and from Laboratory of Soil Microbiology of Nairobi University (strain USDA110).
Isolates were cultured in Yeast Extract Mannitol (YEM) broth at 28°C for one week. Soybean seeds were surface sterilized in 70% ethanol for 30s and then in sodium hypochlorite solution (0.25% available chlorine) for 3 min and washed with sterile distilled water [17]. These seeds were pregerminated for 72 h on water agar (0.7 w/v) and after germination seedlings were planted at the rate of two seedlings in 250 ml pots containing 200 g of washed and autoclaved sand. 1 mL of the mixture, with approximately a density of 108 viable rhizobia per ml was supplied to the germinated soybean seeds (variety TGX 1910 14F) at planting. After seven days, seedlings were thinned to one plant per pot.
Plants were watered every two days with 25 ml of a sterile Jensen's nutrient solution (N-free nutrient solution) with the following concentrations: 0.2 gL-1 K2HPO4; 0.1 gL-1 FeCl3∙6H2O; 0.2 gL-1 NaCl; 1 gL-1 CaHPO4; 2.86 mgL-1 H3BO3; 0.2 gL-1 MgSO4∙7H2O; 2.03 mgL-1 MnSO4∙4H2O; 0.08 mgL-1 CuSO4∙5H2O; 0.09 mgL-1 Na2MoO4∙H2O and 0.22 gL-1 ZnSO4∙7H2O [18].
Nodulation was evaluated after 4 weeks of cultivation. Nodules were scored using 0 to 5 scoring system where 0=no nodules, 1=1 to 5 nodules (rare), 2=6 to 10 nodules (few), 3=11 to 20 nodules (moderate), 4=21 to 50 nodules (abundant) and 5=>50 nodules (extra abundant) [19]. The greenness of plants was assessed by comparing the inoculated plant color with the uninoculated plants color as follow: 1=dark green, 2=light green and 3=yellowish [19]. The analysis of variance was done with Statistical Analysis System (SAS) and the means were compared using Student-Newman-Keuls difference test.
Effectivity of isolates on sterilized soil
After authentication, 28 best isolates were used in a greenhouse experiment to test their symbiotic effectiveness in pots containing 2 kg sterile soil. A completely randomized experimental design with 36 treatments (28 isolates, 4 references strains, control without N and control with mineral N), replicated four times, was used for this experiment. Before sowing, the seeds were disinfected as was previously described. Three seeds were then planted per pot and thinned to one plant per pot at 7 days after sowing. At sowing 1 ml of bacterial suspension, containing about 108 cells, was used to inoculate each seedling. Apart from “plus N control treatment”, all treatments were irrigated twice per week with the N-free nutrient solution. For plus N control treatment, 0.05% (w/v) KNO3 was added weekly in the nutrient solution [20].
At 6 weeks after sowing, number of leaves, plant height, shoots and root dry matter, nodulation (numbers of nodules and nodule dry matter) were assessed. Shoots, roots and nodules dry matter were evaluated by drying the material at 65°C for 72 hours. Total N content in the shoots was measured using the Kjeldahl method [21]. N uptake was estimated by multiplying total N content by the dry aerial biomass.
All data were subjected to Analysis of Variance (ANOVA) using SAS software version 9.2. The statistical significance was determined at P ≤ 0.05 using Student-Newman-Keuls test. Compositional analysis of the strains communities was performed using multivariate cluster analysis in Minitab software version 14.1.
Effectivity of isolates on non-sterilized soil
Soil samples were collected from the three agro ecological zones at a depth of 0-20 cm. Most probable numbers of compatible rhizobia with soybean in soils were determined using the plant infection technique [22]. The soils were characterized for their chemical characteristics as follows. Nitrogen was determined using Kjeldahl method [21]. Total carbon content was assessed by the Walkley-Black acid digestion method [23]. Total and available phosphorus were extracted using Bray 1 method and were measured using molybdenum blue colorimetry. The Cation Exchange Capacity (CEC) was assessed according to Metson method [24]. The pH was measured in water (soil: water ratio 1:2.5) and in KCl (1:2.5) using a digital pH meter. The number of rhizobia in the soil was assessed by Most Probable Number (MPN) method [15].
A composite soil sample, from each of experimental fields sampled, was then done per AEZ. Two kilograms of the composite soil sample were weighted per pot. Eight inoculation treatments (control, LMSEM 22, LMSEM 25, LMSEM 53, LMSEM 97, LMSEM 101, FA3 and STM3043), three different soils composite soil samples of AEZ 3: S1, AEZ 4: S3 and AEZ 5: S4) arranged in completely randomized block design and replicated three times were established in the greenhouse. Three seeds of soybean variety TGX 1910 14 F were sown per pot and inoculated with 1 ml (about 108 cells) of bacterial suspension. The plants were thinned to one plant per pot at seven days after sowing and were watered every two days.
At 6 weeks after sowing, shoots and root dry matter, numbers of nodules and nodule dry matter were assessed. The collected data were subjected to analysis of variance using SAS software.
Isolation, Identification of bacteria and authentication of isolates
One hundred and two root modulating bacteria were isolated from the nodule of soybean plant, 36 originating from the AEZ 3, 36 from AEZ 4 and 30 from AEZ 5. All strains were Gram-negative. They did not absorb red color when cultured in YEMA containing congo red dye. Characterization of the isolates, based on growth test, showed that 72% were fast growers (colonies visible after three days of incubation) and 28% were classified as slow growers (longer than five days). Reaction on YEMA with BTB distinguished 13% alkaline producers and 87% acid producers.
Authentication test showed that 80 isolates were able to form nodule on soybean root. Significant differences in leaf color and nodule score were observed (P<0.001) among plants inoculated with different strains and non-inoculated control (data not presented). The highest nodules scores were obtained with the isolates LMSEM 22 (4.8), LMSEM 101 (4.8), LMSEM 97 (3.2), LMSEM 46 (3.1) and LMSEM 25 (2.8). The isolates LMSEM 101 and LMSEM 22 had better nodule score than the four reference strains. About the leaf color score, 47% of isolates induced a dark green color, 25% light green color and 28% gave a yellowish color.
Effectivity of isolates on sterilized soil
The effects of the different isolates on the nodulation and growth of soybean are presented in Table 2.
| Trains | Origin | Height | LeafNb | NodNb | NodDW | ShootDW | RootDW | N uptake |
|---|---|---|---|---|---|---|---|---|
| LMSEM1 | AEZ 5 | 46c | 12c | 3f | 36e | 4.18d | 1.48b | 3.2c |
| LMSEM 2 | AEZ 4 | 57b | 11c | 11d | 27f | 4.40d | 1.54b | 3.36b |
| LMSEM 3 | AEZ 5 | 57b | 10d | 5e | 27f | 4.11d | 1.47b | 3.14c |
| LMSEM 7 | AEZ 4 | 63ab | 11c | 3f | 18g | 4.38d | 1.53b | 3.35b |
| LMSEM17 | AEZ 5 | 56b | 11c | 18d | 51d | 5.38c | 1.78b | 4.1b |
| LMSEM20 | AEZ 3 | 49b | 14b | 16d | 106b | 5.18c | 1.73b | 3.95b |
| LMSEM22 | AEZ 4 | 65ab | 13b | 30b | 548a | 7.41a | 2.29a | 5.62a |
| LMSEM24 | AEZ 4 | 67ab | 11c | 7e | 16g | 5.47c | 1.8ab | 4.17b |
| LMSEM25 | AEZ 4 | 62ab | 13b | 25c | 66d | 6.19b | 1.98ab | 4.71ab |
| LMSEM32 | AEZ 4 | 59b | 10c | 4f | 87c | 4.95c | 1.67b | 3.78b |
| LMSEM37 | AEZ 3 | 46c | 12c | 8e | 16g | 4.84d | 1.65b | 3.69b |
| LMSEM44 | AEZ 5 | 48b | 10c | 5e | 17g | 4.03d | 1.44c | 3.09c |
| LMSEM46 | AEZ 4 | 58b | 11c | 8e | 48e | 4.38d | 1.53b | 3.35b |
| LMSEM47 | AEZ 4 | 52b | 12c | 23c | 136b | 4.85d | 1.65b | 4.75ab |
| LMSEM48 | AEZ 5 | 48b | 12c | 6e | 24f | 3.77e | 1.38b | 2.89d |
| LMSEM50 | AEZ 3 | 60ab | 11c | 5e | 29f | 5.65c | 1.85ab | 4.3b |
| LMSEM53 | AEZ 3 | 54b | 16a | 12d | 76c | 6.12b | 1.97ab | 4.65ab |
| LMSEM77 | AEZ 3 | 70a | 11c | 3f | 17g | 6.01b | 1.94ab | 4.57ab |
| LMSEM78 | AEZ 5 | 69a | 12c | 4f | 57d | 5.97c | 1.93ab | 4.54ab |
| LMSEM81 | AEZ 3 | 67ab | 11c | 3f | 56d | 6.02b | 1.94ab | 4.58ab |
| LMSEM85 | AEZ 4 | 57b | 12c | 9e | 41e | 4.87d | 1.65b | 3.72b |
| LMSEM86 | AEZ 4 | 63ab | 10c | 3f | 16g | 4.66d | 1.6b | 3.56b |
| LMSEM93 | AEZ 4 | 63ab | 12c | 4f | 38e | 4.45d | 1.55b | 3.4b |
| LMSEM96 | AEZ 5 | 66ab | 11c | 3 | 16g | 4.12d | 1.47c | 3.15c |
| LMSEM97 | AEZ 5 | 62ab | 12c | 28b | 276b | 6.48b | 1.95ab | 5.03a |
| LMSEM98 | AEZ 3 | 60ab | 12c | 12d | 66d | 5.33c | 1.77b | 4.06b |
| LMSEM99 | AEZ 3 | 56b | 11c | 20c | 41e | 4.58c | 1.58b | 3.5b |
| LMSEM101 | AEZ 3 | 65ab | 13b | 46a | 406ab | 6.50b | 2.06a | 4.94ab |
| IRAT FA3 | France | 61b | 12c | 11d | 17g | 5.90b | 1.9ab | 4.9ab |
| STM3043 | France | 64ab | 13c | 29b | 133b | 6.46b | 2.05a | 4.91ab |
| Control N- | - | 71a | 12c | 13d | 98c | 5.83c | 1.89ab | 5.44a |
| Control N+ | - | 43c | 9d1 | 0g | 0h | 2.87f | 1.15d | 2.22d |
| Probability | - | <0.0001*** | <0.0001*** | <0.0001*** | <0.0001*** | <0.0001*** | <0.0001*** | <0.0001*** |
| CV | - | 5 | 6 | 3 | 2 | 0.1 | 0.05 | 0.8 |
Note: The means with the same alphabetic letters on the same column are not significantly different (P>0.05) according to the Newman-Keuls test; LeafNb: Number of Leaves (number plant-1) ; NodNb: Nodule Number (number plant-1); NodDW: Nodule Dry Weight (mg plant-1); ShootDW: Shoot Dry Weight (g plant-1); RootDW: Root Dry Weight (g plant-1); N uptake (gN plant-1); Control N+: Control with N; Control N-: Control without N.
Table 2: Effects of the different isolates in the nodulation and growth of soybean.
Nodulation: Results showed that all the isolates including the four reference strains formed nodules on soybean root with significant differences (p<0.01) but the non-inoculated controls with and without mineral nitrogen produced no nodules. Nodule number ranged from 0 (control) to 46 (LMSEM 101). The five best strains were: LMSEM 101, LMSEM 22, STM3043, LMSEM 97 and LMSEM 25. A significant different was also found on nodule dry weight which varied from 0 (control) to 406 mg per plant (S22). The five strains with the best nodule dry weight were: LMSEM 22, LMSEM 101, LMSEM 97, LMSEM 46 and STM3043. The isolates LMSEM 101 and LMSEM 22 outperformed all the four reference strains for nodule number and nodule dry weight respectively.
Shoot dry matter:A significant difference was found between strains for shoot dry matter. The five strains with higher shoot dry matter values included successively strains LMSEM 22, LMSEM 101, LMSEM 97, STM3043 and LMSEM 25. The isolated strains LMSEM 22, LMSEM 101 and LMSEM 97 produced more biomass than the reference strains. Isolates LMSEM 22, LMSEM 101, LMSEM 97, LMSEM 25 and two references strains (STM3043 and IRAT FA3) significantly promoted plant biomass which was like the treatment receiving mineral nitrogen.
Nitrogen uptake in shoot biomass: There were significant differences (P<0.001) in plant nitrogen uptake in aerial biomass among the treatments, with values ranging from 2.2 gN plant-1 (control without mineral N) to 5.6 gN plant-1 (LMSEM 22). The nitrogen accumulation in the shoot biomass of plant inoculated with strains LMSEM 22 and LMSEM 101 were similar to that of nitrogen fertilizer control. These two endogenous isolates outperformed the two reference strains.
Plant height: Most of the isolates significantly promoted the height of soybean plant compared to the control without nitrogen. Plant height ranged from 43 cm (control without nitrogen) to 71 cm (control+nitrogen). It was higher with isolates LMSEM 101, LMSEM 22, LMSEM 97 and LMSEM 25. Isolate LMSEM 101 outperformed all the reference strains but improved soybean height as much as the control with nitrogen. On the other hand, isolates LMSEM 1, LMSEM 37, LMSEM 44, LMSEM 126 and LMSEM 128 did not significantly improve the height of soybean plant compared to control without nitrogen.
Number of leaves per plant: Higher number of isolates showed positive effect and increased the number of leaves of soybean plant which varied from 9 (control without N) to 16 (LMSEM 101). The isolates LMSEM101, LMSEM 22, LMSEM 97 and LMSEM 25 surpassed all the reference with a gain ranging from 17% to 35% but were like the control with mineral nitrogen. Isolates LMSEM 3, LMSEM 32, LMSEM 44, LMSEM 86, LMSEM 108, LMSEM 115, LMSEM 126 and LMSEM 127 did not significantly increase plant height compared to the control without nitrogen.
Root dry weight per plant: The root dry weight of plants inoculated with the different isolates and reference strains was significantly higher than that of control without N. It varied from 1.15 g plant-1 (control without mineral N) to 2.29 (LMSEM 22). But isolates LMSEM 101, LMSEM 22, LMSEM 97 and LMSEM 25 had root dry weight superior to that of reference strains but like that of the positive control (control+N).
Strains community composition: Based on nodulation and growth parameters, five groups were obtained from hierarchical classification of strains (Figure 2). The first group (G1) was composed of fifteen isolates which were LMSEM 2, LMSEM 46, LMSEM 7, LMSEM 96, LMSEM 93, LMSEM 32, LMSEM 86, LMSEM 85, LMSEM 98, LMSEM 3, LMSEM 17, LMSEM 99, LMSEM 20, LMSEM 37 and LMSEM 47. The second (G2) gathered two reference strains (USDA110 and STM3045) and five isolates namely LMSEM 77, LMSEM 81, LMSEM 78, LMSEM 24 and LMSEM 50. The third (G3) consisted of two reference strains (IRAT FA3 and STM3043), the control with mineral nitrogen and three isolates (LMSEM 25, LMSEM 53 and LMSEM 97). The fourth group (G4) assembled the control without nitrogen and three isolates which were LMSEM 1, LMSEM 48 and LMSEM 44. The fifth group (G5) gathered the isolates LMSEM 22 and LMSEM 101.
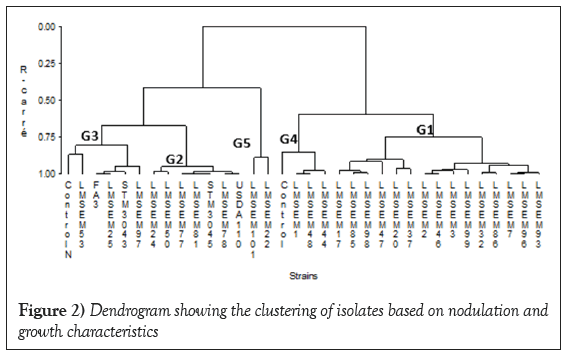
Figure 2: Dendrogram showing the clustering of isolates based on nodulation and growth characteristics.
The result of the Principal Components Analysis (PCA) showed that the first two principal components explained 98.40% of the variation; so, a two (2) dimensions (axes) solution is best for this data set. The first principal component was a measure of the bad quality of shoot dry weight, root dry weight and N uptake. The second principal component was a measure of the good quality of height, number or leaf, number of nodule and nodule dry weight. Projection of strains communities in system axes (Figure 3) revealed that community of strains of group 1, group 2 and group 3 were opposed to community of strains of group 4 and group 5 when the first component was considered. The community of strains of group 3 and group 5 were opposed to community of strains of group 1, group 2 and group 4 when the second component was considered. Moreover, most of strains coming from different agro ecological zone do not appear in well-defined cluster. They were dispersed among the clusters.
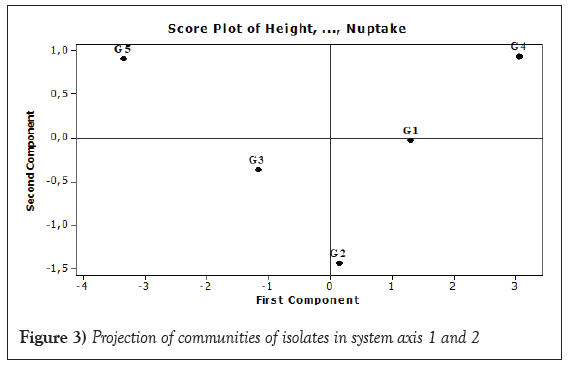
Figure 3: Projection of communities of isolates in system axis 1 and 2.
Effectivity of isolates on unsterilized soil
Assessment of the fertility level of soils used and number of native rhizobia: Chemical analysis of soils collected in the three agro ecological zones investigated shows that these soils are degraded. They are poor in nutrients (nitrogen, organic carbon and available phosphorus) and the CEC is also low (Table 3). On the other hand, the soils are near-neutral soils with pH (H2O) values ranging between 6.5 and 6.8. Endogenous rhizobia infective for soybean were present in all the fields. The numbers of native rhizobia were 384, 390 and 387 rhizobia g-1 soil respectively in AEZ 3, AEZ 4 and AEZ 5.
Nodulation, shoot dry matter and root dry matter: All isolates and the reference strains formed nodules and induced plant shoot and root biomass more than the control (Figure 4). Soil type significantly affected the performance of the different strains and the highest values were generally obtained on the soils collected in AEZ 3 and AEZ 4. The isolates LMSEM 22, LMSEM 101 and LMSEM 97 outperformed the two reference strains in all types of soil.
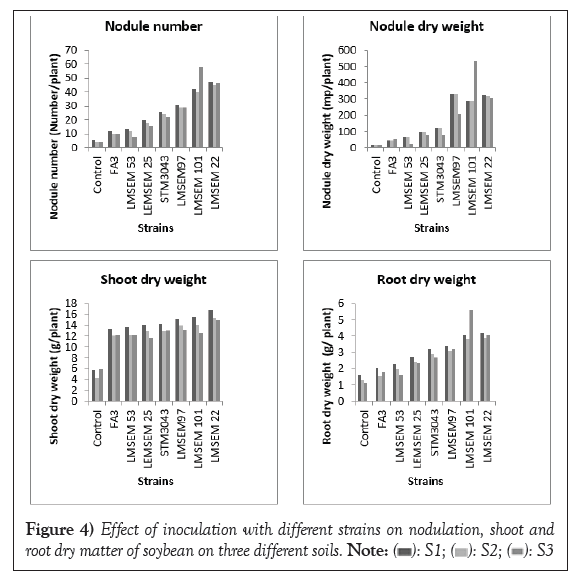
Figure 4: Effect of inoculation with different strains on nodulation, shoot and
root dry matter of soybean on three different soils. 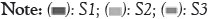 .
.
Isolation and authentication of isolates
In the present study 102 presumptive rhizobia strains were isolated from the root nodule of soybean grown in three AEZs of Benin. All presumptive rhizobia strains isolated were identified as gram negative with no absorption of congo red. Most of them formed colonies after three days and were acid producers and fast grower. These results confirmed that most of the isolates were fast grower (acid producer and growth occurring 72 h after incubation). Similar results were obtained by Singh et al. [25], Costa et al., [26] on soybean, pigeon pea and Bambara groundnut respectively. Nevertheless, Somasegaran et al. [22], indicated that soybean is generally associated to slow grower rhizobia [27].
On the 102 presumptive rhizobia, 80 isolates were able to form nodule on soybean root. This indicates the importance of authentication test (nodulation tests) following the Koch’s postulates on bacterial isolates sampled from the root nodules. This test is very important to ensure that the presumptive rhizobia are ‘true’ symbionts of the soybean plants considering that nodules can be occupied by various bacteria which are not able to induce nodules or fix N2 [28].
A significant difference was found in green color of plants inoculated with different presumptive rhizobia strains isolated compared to the control and most of isolates improved green color of soybean. Indeed, plant color is a potential indicator for nitrogen status of crops [29]. Moreover, nitrogen is the principal component of chlorophyll that confers green color to the plant and photosynthesis is also dependent on nitrogen assimilation to make or essential structures or molecules [30]. So, nitrogen deficiency leads to loss of green color in the leaves, reduction of leaf area and photosynthesis intensity [1]. Therefore, most of isolates increased nitrogen content of soybean plant compared to control.
Effectivity of isolates
Results showed a significant difference in nodulation of soybean plant inoculated with the different isolates in the sterile media. These statements are consistent with those of Benson et al., [13]. Who showed a considerable variation in nodulation and plant growth among the native isolates in sterile media. Positive (+N) and negative (-N) controls produced no nodules. Similar results were already reported by Wasma et al., [12] who showed that it is essential that both negative (-N) and positive (+N) controls were not nodulated as the former indicates that contamination was minimized.
Inoculation of soybean with the isolates and the reference strains resulted also in variation in height, number of leaves, shoot and root dry weight and nitrogen uptake as shown in Table 3. Endogenous isolates LMSEM 22 and LMSEM 101 outperformed all the reference strains. In addition to LMSEM 22 and LMSEM 101, isolates LMSEM 25, LMSEM 53 and LMSEM 97 were comparable to the reference strains IRAT FA3 and STM 3043 which surpassed the other two reference strains STM3045 and USDA 110. Wasma et al., [12] and Appunu et al., [31]. Also concluded that native rhizobia can be symbiotically more effective than reference strains. Benson et al., [13] came to the same results and showed that isolate BAMKbay8 outperformed the commercial isolate USDA110 and nearly equaled the reference strain KFR 259 in symbiotic performance. The difference in nodulation as well as in growth and symbiotic parameters observed with different endogenous isolates could be due to the difference in the genetic but also in effectiveness of each strain since soil and climatic variations were minimal.
| AEZ 5 (S3) | AEZ 4 (S2) | AEZ (S1) | |
|---|---|---|---|
| pH (H2O) | 6.5a | 6.7a | 6.8a |
| pH (Kcl) | 5.9a | 6.1a | 6.3a |
| Total nitrogen (%) | 0.04a | 0.09a | 0.07a |
| Organic matter (%) | 1.48a | 1.46a | 1.55a |
| Organic carbon (%) | 0.95a | 0.90a | 1.01a |
| Available phosphorus (ppm) | 69.45a | 86.20b | 83.36b |
| Total phosphorus (ppm) | 198.55b | 310.91a | 298.37b |
| CEC (meq/100 g) | 19.26a | 22.1a | 23.5b |
| Most probable number (rhizobia g-1) | 387 | 390 | 384 |
Note: The means with the same alphabetic letters on the same line are not significantly different (P>0.05) according to the Newman-Keuls test.
Table 3: Chemical characteristics and most probable number of native rhizobia of soils (S1, S2, S3) under soybean crop in the three investigated Agro Ecological Zones (AEZ).
Five plant communities, significantly different, were identified on isolates. Moreover, most of strains coming from different agro ecological zones do not appear in well-defined cluster. They were dispersed among the clusters. So, no correlation was not found between the grouping of isolates and the geographical origin. Indeed, isolates from the same origin were found in diverse groups and isolates from different origins were included in the same group. Similar results were obtained by Laurette et al., [27] on Bambara groundnut. However, Aliyu et al., [32] showed that rhizobia strains of different origin could vary in their symbiotic effectiveness.
Results obtained on non-sterile soil showed that isolates LMSEM 22, LMSEM 101 and LMSEM 97 outperformed the two reference strains on all types of soils. These results are consistent with those of Waswa et al., [12] in Kenyan where the native strains outperformed the USDA110 on clay soils in a greenhouse environment. Similar results have also been observed by Karaca et al., [33] in Turkish soils in Phaseolus vulgaris where endogenous strains outperformed the international industry standard strain (CIAT899) in symbiotic efficiency and were recommended for inoculant trials. The number of endogenous Rhizobium ranged between 384 to 390 bacteria g-1 of soil (Table 3). Then, the poor performances of the reference strain compared to the native ones could be due to the development in the soil of endogenous Bradyrhizobium strains which sometimes have antagonistic effects on introduced strains as stated by Mathu et al., [34] whose work has shown that inoculation in soils where endogenous rhizobia strains are strongly present is often unsuccessful. Nevertheless, the success of commercial inoculant could not be due to only the number of viable rhizobia cells but could also depend on the nature of endogenous soil population of rhizobia or the absence of other microbial contaminants [35,36].
Furthermore, soil type significantly affected the performance of the different isolates. The best results were obtained in soils of AEZ 3 and AEZ 4 while the low values were found in AEZ 5 soils. This variation could be attributed to the chemical characteristics of the soils. Indeed, soils of AEZs 3 and 4 had more nutrient contents (nitrogen, carbon, organic matter, available phosphorus, cation exchange capacity) than those of AEZ 5 (Table 3). This confirms the influence of soil type on the efficiency of the rhizobia-legume symbiosis as reported that edaphic factors such as soil pH, phosphorus and nitrogen availability may affect the efficiency of the legume-rhizobia symbiosis [35-38]. Hence, balanced fertilization is important to the success of legumes inoculation. So, promotion of rhizobia inoculation without preliminary soil fertility level diagnosis must be reexamined to consider nutrients balanced [37,39].
Moreover, initial total N soil analyses showed that the soils of AEZs 3 and 4 had respective higher contents of nitrogen of 0.07 and 0.09% than the 0.04% found in the soils of AEZ 5 as illustrated in Table 3. The higher content of starter N could also be a contributing factor to the better performance on the symbiotic partnerships [40].
In conclusion the potential for improving symbiotic nitrogen fixation in soybean through the identification of elite native rhizobia strains adapted to local environmental conditions in Benin. The most effective presumptive isolates of Rhizobium were confirmed to be LMSEM 22 and LMSEM 101 since they outperformed reference strains. Three other isolates, LMSEM 25, LMSEM 53 and LMSEM 97 were compared favorably with the reference strains STM3043 and IRAT FA3. However, these presumptive rhizobia strains need to be firstly characterized and secondly tested in farmer’s field before they are recommended for use as commercial products. These findings underscore the presence of effective endogenous rhizobia strains in Benin, offering promising avenues for enhancing soybean productivity through sustainable agricultural practices.
This work was supported by the University of Abomey-Calavi under the project Productivite des Systems Agriculture-Elevage Intégrés (PROSAEI) of competitive Research Fund Program and the International Atomic Energy Agency (IAEA) under the Technical Cooperation Project BEN/5/007.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Zoundji MCC, Akplo TM, Balogoun I, et al. Screening of endogenous rhizobia strains associated to soybean (Glycine max (L.) Merrill) in Benin for their effectiveness in different types of soils. AGBIR.2024;40(3):1057-1063.
Received: 03-Apr-2024, Manuscript No. AGBIR-24-131274; , Pre QC No. AGBIR-24-131274 (PQ); Editor assigned: 05-Apr-2024, Pre QC No. AGBIR-24-131274 (PQ); Reviewed: 19-Apr-2024, QC No. AGBIR-24-131274; Revised: 26-Apr-2024, Manuscript No. AGBIR-24-131274 (R); Published: 03-May-2024, DOI: 10.35248/0970-1907.24.40.1057-1063
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
