Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2023) Volume 39, Issue 3
The potential protective effect of peppermint (Mentha piperita; Labiatae) water extract was investigated on onion (Allium cepa) root tips that were exposed to cytotoxic and genotoxic insecticides; cypermethrin or malathion. Cypermethrin was tested at 0.019, 0.038 and 0.075 mg/ml and malathion at 0.235, 0.469 and 0.938 mg/ml, based on concentrations used in the field. Parallel doses of 4 µg/ml or 8 µg/ml peppermint extract were applied for each insecticide treatment, while distilled water was used as a control. Exposure time was 8 hours for all treatments. Mitotic Index (MI), Cell Proliferation Kinetics (CPK), percentage of Mitotic Aberrations (MAs) and Nuclear Aberrations (NAs) were evaluated. Both insecticides exerted a significant decline in MI (p<0.001) and mitotic kinetics aberrations, which indicated potent cytotoxicity of both insecticides even at lower doses, where significant reduction of MI (more than 50% below the control) because of prophase imbalance and metaphase arrest. Both insecticides induced a concentration dependent MAs ranged between 19.0 and 29.6%. MAs and TAs of cypermethrin were significant at the high dose, while those caused by malathion were significant at both medium and high doses. Aberrations observed included; bridges, vagrants, laggards, stickiness, multipolar, binucleated cells, micronuclei, lobed, blebbed and vacuolated nuclei. Application of peppermint water extract to both insecticides reduced the cytotoxic and genotoxic capacity of these insecticides.
Herbicide; Cytotoxicity; Genotoxicity; Mitotic index; Mitotic aberrations
Insecticides are chemicals aimed at actively treating plant crops to combat insects. They are internationally recognized as a means of protecting plants and are believed to significantly improve agricultural yields. Cypermethrin and malathion are organophosphorus insecticides widely used in agriculture to control insects. WHO classifies cypermethrin as moderately dangerous (Class II). Cypermethrin and malathion have neurotoxic effects on the peripheral and central nervous systems of mammals and insects [1,2].
The gradual accumulation of cypermethrin and malathion insecticide residues, especially when used indiscriminately, causes environmental pollution and adversely affects the normal lives and genetic resources of plants, animals and humans. Intensive use of malathion polluted food and drinking water [3]. Basiak et al., [4] suggested malathion as a possible human mutagenic/carcinogenic substance, while Demirhan et al., [5] detected a significant increase of chromosomal abnormalities in farmers exposed to pesticides. The clastogenic activity of malathion was confirmed [6–8]. Significant chromosomal abnormalities were induced by low concentrations (1/10 or 33.6 µM of LC50) of cypermethrin [9].
Bioactive compounds are components of medicinal phytochemistry and have the ability to prevent/reverse carcinogenesis in the early stages. Leaf aqueous extract of M. citrifolia reduced chromosomal aberrations indicating antigenotoxic potential [10], while M. piperita and M. arvensis protected irradiated-mice against mutations due to their ability to scavenge free radicals, chelate antioxidant metals, anti-inflammatory properties, as well as enhanced anti-mutagenicity and DNA repair. Curcuma longa (curcumin) was used to reduce chromosomal damage in rats injected with cyclophosphamide [11] and it was also reported to neuroprotect against aluminum stress [12]. Extracts of Rosa canina and Salvia lavandulifolia (whole plant or fruit) were mixed with dimethyl sulfoxide (an organophosphorus pesticide) to neutralize the genotoxicity of the pesticide [13].
Significant anti-genotoxicity of Mentha longifolia and Salvia officinalis extracts has been reported [14]. Pretreatment of mice with Mentha extract stabilized DNA and cell cycle, in addition to antioxidant and MAO-A inhibition [15]. The aqueous extracts of M. piperita ameliorated arsenic toxicity [16], while M. longifolia reduced chromosomal aberrations and DNA damage induced by cyclophosphamide in mouse [17]. Peppermint (M. piperita) showed antimutagenic activity by enhancing error-free DNA repair [18].
The present study was aimed at examining the potential protective effects of the perennial aromatic herb peppermint (M. piperita; Labiatae) on cytotoxic and genotoxic effects of insecticides (cypermethrin and malathion) on onion (A. cepa) root tips.
Extraction of peppermint
Leaves of peppermint (M. piperita) were obtained from a local farm in Al-Ahsa, Saudi Arabia. The leaves were thoroughly cleaned with running tap water, left at room temperature for few hours and then completely dried in an oven at 40°C. Water extract was obtained by 1liter of distilled water was mixed with 8g of leaves powder, left on a shaker for 48 hours, centrifuged at 3000 rpm for 10 min, filtered through filter paper (Whatman No.1) and kept in a refrigerator until used.
Insecticides (Chemicals)
Commercially available Cypermethrin (Devicyper 10% Effective Concentration; EC) and Malathion (57% EC) were used and the range of test doses for each was selected based on the real effective concentration applied in the field to control insects. For cypermethrin the EC is equal to 0.075 mg/ml, 1/2 EC (0.038 mg/ml) and 1/4 EC (0.019 mg/ml). The Malathion tested doses were: EC (0.938 mg/ml), 1/2 EC (0.469 mg/ml) and 1/4 EC (0.235 mg/ml). The control assay was distilled water (0.0).
Bulb preparations and roots growth
Average sized healthy A. cepa bulbs were obtained from a local market, Al-Ahsa, Saudi Arabia. Dead outer scales were removed carefully without injuring root primordia. The roots were grown from bulbs on glass containers filled with distilled water that was renewed every day. When roots were about 1.5 to 2.0 cm long, the bulbs were transferred to test concentrations of both insecticides.
Cytotoxicity assay
Five groups of bulbs were transferred to clean containers filled with cypermethrin test concentrations; 0.0, 0.019, 0.038, and 0.075 mg/ml; cypermethrin test concentrations mixed with 4 µg/ml peppermint extract; and a third group of cypermethrin test concentrations mixed with 8 µg/ml peppermint extract. Similar assays were prepared for malathion including the test concentrations, 0.0, 0.235, 0.469 and 0.938 mg/ml plus two groups of test concentrations mixed with 4 µg/ml or 8 µg/ml peppermint extract. The roots were exposed to the test solutions for 8 hours, washed with running water and distilled water. Roots were excised from treated bulbs, fixed in alcohol:glacial acetic acid (3:1) for 24 hours, transferred to 70% alcohol and stored at 4°C in a refrigerator until further use. For cytological observations, the roots were washed with distilled water, hydrolyzed in 1N HCl in a water bath at 60°C for 10 minutes. Roots tips (1-2 mm) were excised stained in 2 drops of Aceto-orcein on clean slides for 20-30 minutes and then squashed with a glass rod.
Cytogenetic analysis
Five slides were prepared from each treatment and meristematic cells (about 1000/slide) were examined under a microscope coupled with a camera (Zeiss Axiocam, Germany). Photomicrograph shots were taken from the provided digital camera. The Mitotic Index (MI) expressed as a percentage following the method of Özmen et al., [19]. Cell Proliferation Kinetics (CPK) frequencies were calculated following the method of Lukaszewicz et al., [20]. Chromosomal and Mitotic Aberrations (MAs) were recorded as percentage of mitotic aberrations. These aberrations include; bridges, stickiness, laggards, vagrants, polyploidy, multipolar and spindle disturbance. The chromosomal and mitotic aberrations were calculated as follows:
MAs (%)=(total number of cells with abnormalities/total number of analysed cells) × 100.
Nuclear Abnormalities (NAs) of interphase include; micronuclei (MN) that were identified following the method [21]. Other NAs include; Binucleated cells (BN), Blebbed Nuclei (BL), Lobed Nuclei (LB) and Vacuolated Cells (VC) that were identified following the method of Nwani et al., [22]. The dividing cells and aberration types were grouped according to the modified A. cepa test introduced by Fiskesjo [23]. These indicators were calculated according to the following formulas:
% frequency of mitotic aberrations (Mas)=TAs × 100/TDC
% frequency of total aberrations (TAs)=TAs × 100/TC
Where:
TC=Total (interphase+mitotic) cells, and TDC=total dividing cells.
Statistical analysis
Results included; MI, MAs% and TAs% were expressed as means and standard errors of triplicate per treatment. Analysis of Variance (One-way ANOVA) was used to find significant differences between the control and treated groups. Comparisons between the control and treated groups were done using the Post-Hoc, Dunnett Multiple Comparison test. Statistical analysis was performed using SPSS version 16 and the significance was accepted at P<0.05, P<0.01, P<0.001.
Mitotic index and cell proliferation kinetics
The analysis of A. cepa root cells treated separately with cypermethrin or malathion without/mixed with peppermint extract for 8 hr exhibited a significant decline in MI for all concentrations (Figures 1 and 2). The MI in cells treated with cypermethrin ranged between 4.4 and 5.17%, while that of malathion was between 4.07 and 5.23%, which was significantly different from the control (17.0% at p<0.001). Addition of peppermint extract to insecticides either showed no effect on MI or induced a slight decline compared to insecticides.
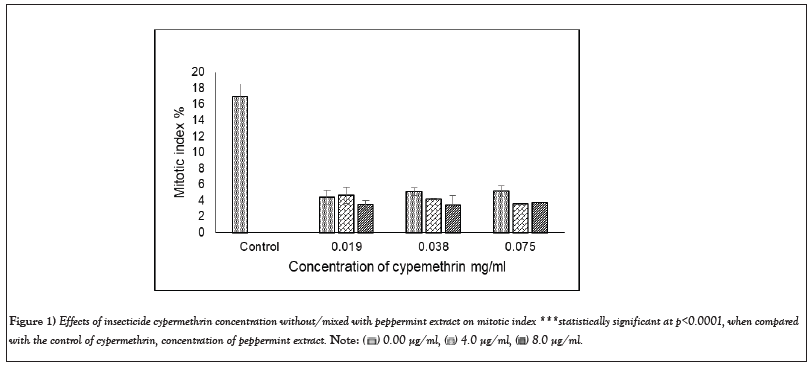
Figure 1: Effects of insecticide cypermethrin concentration without/mixed with peppermint extract on mitotic index ***statistically significant at p<0.0001, when compared
with the control of cypermethrin, concentration of peppermint extract. 
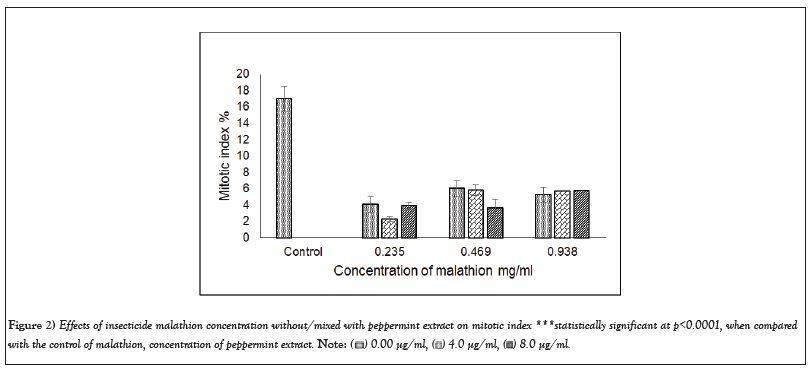
Figure 2: Effects of insecticide malathion concentration without/mixed with peppermint extract on mitotic index ***statistically significant at p<0.0001, when compared
with the control of malathion, concentration of peppermint extract. 
CPK showed dramatic variations between the control and cypermethrin treatments (Figure 3). Cypermethrin decreased the prophase cells percentage (26%-30%) of A. cepa root cells compared to the control (56.0%). Addition of peppermint extract (4 µg/ml or 8 µg/ml) increased prophase cells at the lowest concentration of cypermethrin (46 or 36% at 0.019 mg/ml), but further decreased it at higher concentrations (30 or 25% at 0.038 mg/ml; 6 or 25% at 0.075 mg/ml, respectively). In contrary, cypermethrin increased the metaphase cells percentage (60% at 0.019 mg/ml, 54% at 0.038 mg/ml and 40% at 0.075 mg/ml) compared to the control (20%). Addition of 4 µg/ml peppermint extract reversed (mirror image) the effect of cypermethrin, where the metaphase index decreased to 39% at 0.019 mg/ml then increased gradually to 45 and 59% in the medium and high concentrations, while 8 µg/ml peppermints extract increased the metaphase index to approximately the same level in all cypermethrin concentrations. Cypermethrin decreased the anaphase index (between 1 and 6%), but slightly increased by the addition of peppermint extract. Cypermethrin also increased the telophase index, but the addition of peppermint extract caused slight changes of telophase index.
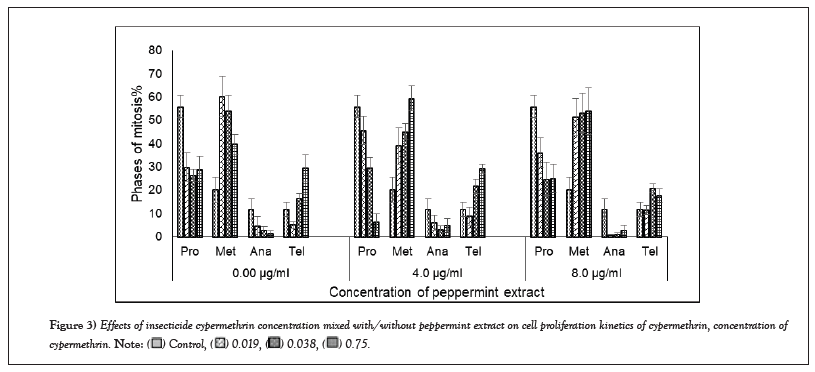
Figure 3: Effects of insecticide cypermethrin concentration mixed with/without peppermint extract on cell proliferation kinetics of cypermethrin, concentration of
cypermethrin. 
Malathion decreased the prophase index to 36% at 0.235 mg/ml, 12% at 0.469 mg/ml and 14% at 0.983 mg/ml compared to the control (56.0%). Malathion significantly increased the metaphase index (57, 74 and 70%, successively) compared to the control (20%). However, on addition of 4 µg/ml or 8 µg/ml peppermint extract, the metaphase index increased to 73% or 79% at 0.235 mg/ml, respectively. Malathion also decreased the anaphase index (8-12%). The telophase index ranged between 2 and 13% (Figure 4).
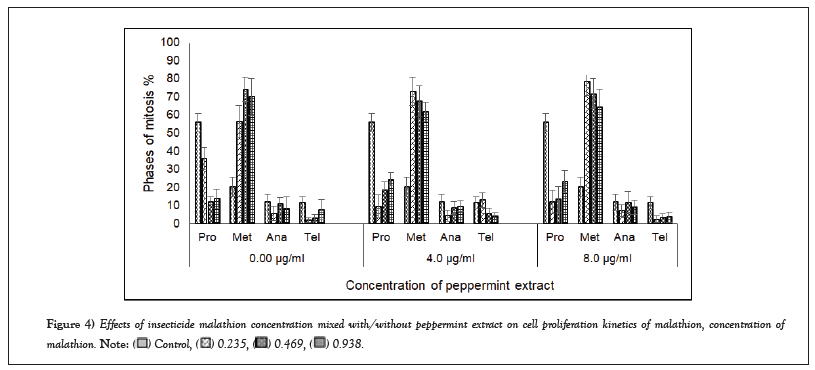
Figure 4: Effects of insecticide malathion concentration mixed with/without peppermint extract on cell proliferation kinetics of malathion, concentration of
malathion. 
Cytological abnormalities
Cytological abnormalities in A. cepa root tips caused by exposure to cypermethrin or malathion treatments for 8 hr were classified into Mitotic Aberrations (MAs; Tables 1 and 2) and Nuclear Abnormalities (NAs; Tables 3 and 4) that were observed in the interphase nuclei. MAs index of the control was 0.4%, while the total NAs was 0.4. Cypermethrin or malathion treatments without/mixed with peppermint extract showed mitotic and nuclear aberrations compared to the control. The most reported MAs were bridges, laggards, vagrants, sticky metaphase multipolar and spindle disturbances, while NAs were micronuclei, binucleated cells, blebbed nuclei, lobed nuclei and vacuolated nuclei. MAs induced by cypermethrin (Table 1) varied between 20% at 0.019 mg/ml and 0.038 mg/ml and 29.6% of the dividing cells at 0.075 mg/ml (significant at p<0.01).
| Treatments | mg/ml | Br | La | Va | St | Pp | Mp | Sd | MAs% |
|---|---|---|---|---|---|---|---|---|---|
| Control | 0.0 | 0.0 ± 0.00 | 0.2 ± 0.32 | 0.2 ± 0.32 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.2 ± 0.32 | 0.42 ± 0.43 |
| 0.0 µg/ml peppermint extract | 0.019 | 6.2 ± 2.42 | 0.4 ± 0.39 | 0.0 ± 0.00 | 2.2 ± 2.76 | 0.0 ± 0.00 | 0.4 ± 0.63 | 0.4 ± 0.39 | 20.5 ± 1.88 |
| 0.038 | 8.6 ± 4.26 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.4 ± 0.63 | 0.0 ± 0.00 | 0.4 ± 0.39 | 0.0 ± 0.00 | 20.0 ± 7.54 | |
| 0.075 | 12.6 ± 5.76 | 0.4 ± 0.39 | 0.2 ± 0.32 | 0.6 ± 0.63 | 0.2 ± 0.32 | 0.0 ± 0.00 | 0.0 ± 0.00 | 29.6 ± 13.74** | |
| 4 µg/ml peppermint extract | 0.019 | 3.4 ± 2.21 | 0.2 ± 0.32 | 0.4 ± 0.39 | 0.4 ± 0.63 | 0.2 ± 0.32 | 0.6 ± 0.39 | 0.2 ± 0.32 | 6.93 ± 5.40 |
| 0.038 | 7.8 ± 1.53 | 0.2 ± 0.32 | 0.0 ± 0.00 | 1.2 ± 1.27 | 0.0 ± 0.00 | 0.2 ± 0.32 | 0.0 ± 0.00 | 25.5 ± 4.10** | |
| 0.075 | 10.6 ± 3.66 | 0.8 ± 0.77 | 0.0 ± 0.00 | 0.2 ± 0.32 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.4 ± 0.39 | 35.6 ± 10.4*** | |
| 8 µg/ml peppermint extract | 0.019 | 4.4 ± 3.66 | 0.6 ± 0.63 | 0.0 ± 0.00 | 0.4 ± 0.39 | 0.0 ± 0.00 | 0.2 ± 0.32 | 0.2 ± 0.32 | 18.2 ± 10.2 |
| 0.038 | 5.8 ± 3.29 | 0.0 ± 0.00 | 0.0 ± 0.00 | 2.8 ± 3.65 | 0.0 ± 0.00 | 0.2 ± 0.32 | 0.0 ± 0.00 | 24.87 ± 9.44* | |
| 0.075 | 3.4 ± 2.32 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.2 ± 0.32 | 11.53 ± 7.86 |
Note: Br=Bridges, La=Laggard, Va=Vagrant, St=Sticky, Pp=Polypoid, Mp=Multipolar, Sd=Spindle disturbance. MAs%=Percentage of mitotic aberrations. ANOVA test:*Statistically significant at p<0.05, **Statistically significant at p<0.01, ***Statistically significant at p<0.0001 compared to the control.
Table 1: Mitotic chromosomal aberrations in cells of A. cepa root-tips exposed to cypermethrin (mg/ml) without/mixed with peppermint extract.
| Treatments | mg/ml | Mn | Bn | Bl | Ln | Vn | TNAs | TAs% |
|---|---|---|---|---|---|---|---|---|
| Control | 0.0 | 0.2 ± 0.32 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.2 ± 0.32 | 0.4 ± 0.63 | 0.1 ± 0.15 |
| 0.0 µg/ml peppermint extract | 0.019 | 1.0 ± 0.71 | 1.4 ± 1.18 | 0.6 ± 0.63 | 1.2 ± 0.59 | 1.6 ± 1.07 | 5.8 ± 1.76 | 1.5 ± 0.46 |
| 0.038 | 0.6 ± 0.39 | 3.2 ± 2.09 | 1.0 ± 1.00 | 0.0 ± 0.00 | 1.2 ± 1.53 | 6.0 ± 3.35 | 1.8 ± 0.74 | |
| 0.075 | 1.8 ± 1.53 | 3.6 ± 2.04 | 0.2 ± 0.32 | 2.6 ± 1.07 | 2.4 ± 0.81 | 10.6 ± 4.02* | 2.8 ± 1.32*** | |
| 4 µg/ml peppermint extract | 0.019 | 1.20 ± 1.16 | 1.2 ± 1.27 | 1.8 ± 0.92 | 0.2 ± 0.32 | 2.6 ± 2.04 | 7.0 ± 5.70 | 1.0 ± 0.21 |
| 0.038 | 0.8 ± 0.32 | 3.0 ± 1.32 | 2.4 ± 1.28 | 0.4 ± 0.63 | 1.6 ± 1.47 | 8.2 ± 2.09 | 2.0 ± 0.26* | |
| 0.075 | 1.6 ± 0.81 | 6.0 ± 2.00 | 3.8 ± 1.36 | 1.8 ± 0.77 | 4.201 ± 0.69 | 16.2 ± 4.07*** | 3.0 ± 0.54*** | |
| 8 µg/ml peppermint extract | 0.019 | 1.2 ± 0.92 | 2.0 ± 1.32 | 1.8 ± 1.76 | 0.4 ± 0.39 | 0.8 ± 1.27 | 6.2 ± 3.26 | 1.3 ± 0.62 |
| 0.038 | 1.20 ± 1.53 | 1.4 ± 1.84 | 0.0 ± 0.00 | 2.6 ± 2.98 | 0.0 ± 0.00 | 5.2 ± 6.25 | 1.3 ± 0.80 | |
| 0.075 | 1.33 ± 0.76 | 1.2 ± 0.92 | 0.8 ± 1.27 | 2.2 ± 1.76 | 3.4 ± 1.63 | 8.93 ± 3.66 | 1.6 ± 0.71 |
Note: Mn=Micronuclei, Bn=Binuclei, Ln=Lobed nuclei, Bl=Blebbed nuclei, Ln=Lobed nuclei, Vn=Vacuolated nuclei, TNAs=Total nuclear abnormalities, TAs%=Total aberrations percentage. ANOVA test:*Statistically significant at p<0.05, **Statistically significant at p<0.01, *** Statistically significant at p<0.001 compared to the control.
Table 2 : Nuclear aberrations in cells of A. cepa root-tips exposed to cypermethrin (mg/ml) mixed with/without peppermint extract.
Cypermethrin induced chromosomal bridges aberrations and it was concentration dependent, while sticky chromosomes were caused only by the lowest concentration (0.019 mg/ml). Addition of 4 µg/ml of peppermint extract reduced both chromosomal bridges aberrations and sticky chromosomes. MAs were also reduced at the lower concentration, but increased MAs at the medium and higher cypermethrin concentrations mixed with 4 µg/ml of peppermint extract. Addition of 8 µg/ml of peppermint extract reduced MAs at the lower and higher concentrations of cypermethrin (Table 1 and Figures 5a-5o).
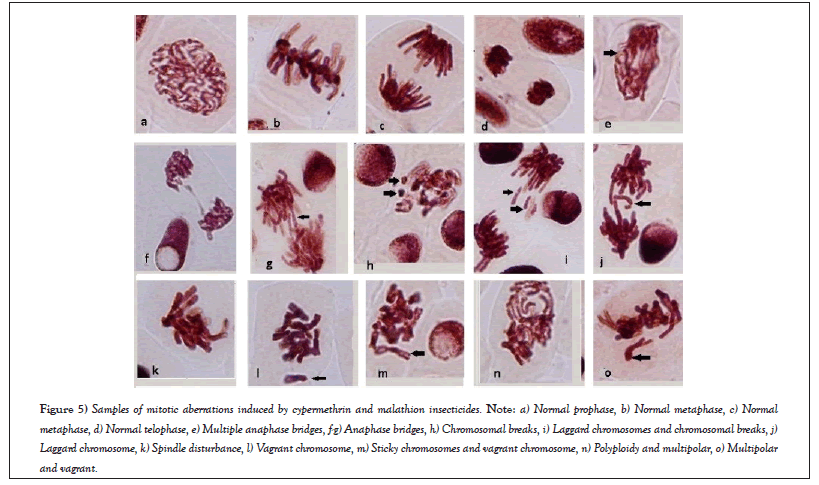
Figure 5: Samples of mitotic aberrations induced by cypermethrin and malathion insecticides. Note: a) Normal prophase, b) Normal metaphase, c) Normal metaphase, d) Normal telophase, e) Multiple anaphase bridges, f-g) Anaphase bridges, h) Chromosomal breaks, i) Laggard chromosomes and chromosomal breaks, j) Laggard chromosome, k) Spindle disturbance, l) Vagrant chromosome, m) Sticky chromosomes and vagrant chromosome, n) Polyploidy and multipolar, o) Multipolar and vagrant.
Cypermethrin induced total nuclear abnormalities, especially binuclei aberrations and it was concentration dependent. Addition of 4 µg/ml of peppermint extract also induced blebbed nuclei and Total Nuclear Aberrations (TNAs), while addition of 8 µg/ml of peppermint extract reduced the binuclei aberrations and it was also concentration dependent (Table 2 and Figures 6a-6p).
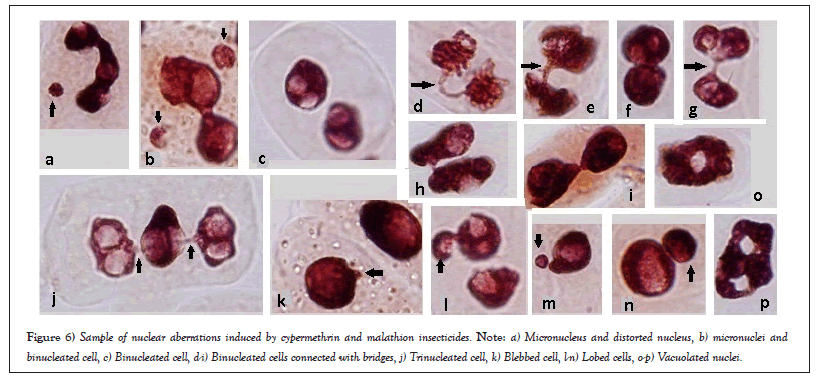
Figure 6: Sample of nuclear aberrations induced by cypermethrin and malathion insecticides. Note: a) Micronucleus and distorted nucleus, b) micronuclei and binucleated cell, c) Binucleated cell, d-i) Binucleated cells connected with bridges, j) Trinucleated cell, k) Blebbed cell, l-n) Lobed cells, o-p) Vacuolated nuclei.
Malathion induced chromosomal bridges aberrations and significantly induced MAs% (ranging between 19 and 28.9%) and it was concentration dependent, though the medium concentration (0.469 mg/ml) also caused more vagrants. Addition of peppermint extract (4 µg/ml) reduced bridges and vagrants at 0.469 mg/ml, but significantly induced MAs% at 0.938 mg/ml of malathion. Addition of 8 µg/ml peppermint extract reduced bridges but induced vagrants and significantly induced MAs% at 0.938 mg/ml of malathion (Table 3).
| Treatments | mg/ml | Br | La | Va | St | Pp | Mp | Sd | MAs% |
|---|---|---|---|---|---|---|---|---|---|
| Control | 0.00 | 0.0 ± 0.00 | 0.2 ± 0.32 | 0.2 ± 0.32 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.2 ± 0.32 | 0.4 ± 0.43 |
| 0.0 µg/ml peppermint extract | 0.235 | 1.4 ± 0.95 | 0.6 ± 0.95 | 3.4 ± 1.78 | 0.2 ± 0.32 | 0.2 ± 0.32 | 0.4 ± 0.28 | 0.4 ± 0.39 | 19.0 ± 10.24 |
| 0.469 | 5.8 ± 2.66 | 1.2 ± 0.92 | 4.8 ± 1.69 | 1.2 ± 0.92 | 0.0 ± 0.00 | 0.8 ± 0.32 | 1.4 ± 1.183 | 28.9 ± 7.1* | |
| 0.938 | 6.0 ± 3.43 | 0.6 ± 0.95 | 1.4 ± 0.81 | 1.0 ± 1.58 | 0.2 ± 0.31 | 0.8 ± 0.92 | 1.2 ± 1.16 | 25.0 ± 10.32* | |
| 4 µg/ml peppermint extract | 0.235 | 3.0 ± 0.71 | 0.0 ± 0.00 | 1.2 ± 0.59 | 0.0 ± 0.00 | 0.4 ± 0.39 | 0.4 ± 0.39 | 0.2 ± 0.32 | 23.2 ± 5.90 |
| 0.469 | 4.0 ± 2.00 | 0.2 ± 0.32 | 1.4 ± 0.95 | 0.8 ± 1.27 | 0.2 ± 0.32 | 0.6 ± 0.95 | 0.2 ± 0.32 | 12.7 ± 6.76 | |
| 0.938 | 5.65 ± 0.29 | 0.0 ± 0.00 | 3.0 ± 0.50 | 10.32 ± 1.44 | 0.0 ± 0.00 | 1.32 ± 0.29 | 1.0 ± 0.50 | 35.8 ± 7.23** | |
| 8 µg/ml peppermint extract | 0.235 | 2.8 ± 1.69 | 0.6 ± 0.63 | 2.0 ± 0.87 | 4.0 ± 2.60 | 0.0 ± 0.00 | 1.6 ± 1.63 | 0.6 ± 0.63 | 28.3 ± 12.9* |
| 0.469 | 6.3 ± 0.59 | 0.0 ± 0.00 | 2.8 ± 1.05 | 2.3 ± 1.26 | 0.0 ± 0.00 | 0.3 ± 0.31 | 0.0 ± 0.00 | 31.7 ± 10.0** | |
| 0.938 | 2.6 ± 1.55 | 1.2 ± 1.16 | 7.0 ± 3.24 | 19.2 ± 11.91 | 0.0 ± 0.00 | 2.6 ± 1.07 | 1.0 ± 1.00 | 50.1 ± 16.76*** |
Note: Br=Bridges, La=Laggard, Va=Vagrant, St=Sticky, Pp=Polypoid, Mp=Multipolar, Sd=Spindle disturbance. MAs%=Percentage of mitotic aberrations. ANOVA test: *Statistically significant at p<0.05, **Statistically significant at p<0.01, ***Statistically significant at p<0.0001 compared to the control.
Table 3 : Mitotic chromosomal aberrations in cells of A. cepa root-tips exposed to malathion (mg/ml) mixed with/without peppermint extract.
Malathion induced various kinds of nuclear abnormalities, especially binucleated cells, vacuolated nuclei, which increased with concentration, but blebbed and lobed nuclei were relatively higher at low concentration and micronuclei were more at high concentration of malathion. The TAs% were significant (p<0.05) at high concentration (0.938 mg/ml) of malathion and at high concentration mixed with 4 µg/ml or 8 µg/ml peppermint extract (p<0.001) compared to the control (Table 4).
| Treatments | mg/ml | Mn | Bn | Bl | Ln | Vn | TNAs | TAs% |
|---|---|---|---|---|---|---|---|---|
| Control | 0.0 | 0.20 ± 0.32 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.20 ± 0.32 | 0.40 ± 0.63 | 0.1 ± 0.15 |
| 0.0 µg/ml peppermint extract | 0.235 | 1.4 ± 1.38 | 1.0 ± 0.87 | 4.0 ± 2.24 | 2.2 ± 1.05 | 0.8 ± 1.27 | 8.6 ± 3.93 | 2.0 ± 1.18 |
| 0.469 | 0.8 ± 0.92 | 1.0 ± 0.00 | 0.6 ± 0.95 | 1.2 ± 0.92 | 2.2 ± 1.05 | 5.8 ± 2.57 | 2.4 ± 0.56* | |
| 0.938 | 2.4 ± 1.47 | 1.4 ± 1.18 | 2.25 ± 1.21 | 1.0 ± 1.00 | 7.6 ± 6.92 | 14.2 ± 9.05** | 3.0 ± 2.01** | |
| 4 µg/ml peppermint extract | 0.235 | 2.0 ± 1.12 | 1.6 ± 1.07 | 0.8 ± 0.92 | 0.2 ± 0.32 | 0.4 ± 0.63 | 5.0 ± 3.08 | 1.0 ± 0.40 |
| 0.469 | 0.0 ± 0.00 | 0.2 ± 0.32 | 0.8 ± 0.77 | 0.0 ± 0.00 | 0.6 ± 0.95 | 1.6 ± 1.63 | 0.9 ± 0.52 | |
| 0.938 | 0.33 ± 0.29 | 1.0 ± 0.00 | 2.32 ± 1.15 | 0.0 ± 0.00 | 7.0 ± 3.78 | 10.6 ± 4.81 | 2.9 ± 2.06** | |
| 8 µg/ml peppermint extract | 0.235 | 1.2 ± 1.16 | 2.8 ± 1.53 | 0.4 ± 0.63 | 1.4 ± 1.07 | 0.2 ± 0.32 | 6.0 ± 2.65 | 1.7 ± 0.72 |
| 0.469 | 2.0 ± 0.50 | 0.0 ± 0.00 | 0.6 ± 0.63 | 2.0 ± 1.32 | 3.4 ± 4.29 | 8.0 ± 5.41 | 1.9 ± 0.69 | |
| 0.938 | 0.4 ± 0.63 | 1.4 ± 1.07 | 2.0 ± 1.50 | 3.8 ± 1.61 | 0.8 ± 1.27 | 8.4 ± 1.63 | 3.8 ± 1.44*** |
Note: Mn=Micronuclei, Bn=Binuclei, Ln=Lobed nuclei, Bl=Blebbed nuclei, Ln=Lobed nuclei, Vn=Vacuolated nuclei, TNAs=Total nuclear abnormalities, TAs%=Total aberrations percentage. ANOVA test: *Statistically significant at p<0.05, **Statistically significant at p<0.01, *** Statistically significant at p<0.001 compared to the control.
Table 4 : Nuclear aberrations in cells of A. cepa root-tips exposed to malathion (mg/ml) without/mixed with peppermint extract.
This study tested the insecticides cypermethrin and malathion based on their effects on Mitotic Index (MI) as a sensitive parameter of cell cycle machinery, tissue proliferation and growth [24,25] as well as a reliable test for detection of cytotoxicity [23]. Increased cytotoxic levels of environmental pollutants can be exposed by decreased rate of MI [26]. Studies carried out by different authors confirmed the cyto-genotoxicity of cypermetrin [27-29]. Malathion toxicity to plants was clearly determined [8,30,31].
As results of this study, all cypermetrin or malathion concentrations exhibited significant decrease in MI compared to the control (Figures 1 and 2). The MI varied between 3.41 and 5.16 that represent a percentage of 20.08-30.39 of the control in cypermethrin, while the MI of malathion ranged between 2.30 and 6.03 which was 13.54-35.48% of the control. The MI decline in both insecticides was approximately similar and its value was not concentration dependent. The highly significant decline of MI revealed the potent cytotoxic effect of both insecticides on A. cepa root cells and in agreement with Sheikh et al. [32]. It was noted that the decline of MI below 50% of the control causes sub-lethal effects and reduction below 22% is lethal. The present record of MI induced by cypermethrin varied between 20.08 and 30.39% and that of malathion varied between 13.54 and 35.48% of the control, which can be considered, at the most optimistic evaluation, as sub-lethal. Similar results of significant MI reduction by more than 50% of the control was detected on treatment of A. cepa cells with 0.137 mg/ml malathion [6].
Genotoxicity is a direct cause of cell cycle delay at the interphase that reduces the number of cells at mitotic phases [33]. Moreover, the reduced MI may also be attributed to the arrest of any of the mitotic phases [34], reduced DNA and protein synthesis, blockage of cell cycle at G2 phase [35] or DNA damage [36-38]. Mitotic phases index (Figure 3) showed dramatic variations between the control and insecticides treatments especially at the prophase and metaphase. Prophase count was 56% compared to other stages and declined to a range between 6% and 46% in cypermethrin and between 10% and 36% in malathion treatments. In contrast, the metaphase scored 20% in the control and dominated in all concentrations of cypermethrin (39%-60%) or malathion (57%-79%).
The imbalance of prophase was significant (p<0.05) in six out of nine cypermethrin treatments compared to the control and all treatments of malathion (p<0.05 and p<0.01). It is relevant to mention that the insignificant changes on prophase were induced by cypermethrin mixed with 4 µg/ml or 8 µg/ml of peppermint extract. It is possible to attribute this to a protective activity of peppermint extract against cypermethrin toxicity on A. cepa root cells. It was reported that cytotoxicity causes irregular mitotic phases where metaphase dominate at the expense of prophase [34]. Similar results of increased metaphase [39] and considerable decrease of anaphase and telophase compared to the control were recorded [40]. The decline in anaphase and telophase, and consequently MI, may be a direct result of mitotic arrest or slow mitosis due to onset obstruction of prophase [41]. The prevalence of metaphase stage induced by cypermethrin and malathion may result from metaphase arrest as a consequence of toxicity [34], failure to build spindle apparatus and microtubules [42] or delay of movement from metaphase to anaphase due to metaphase abnormalities [20].
The frequency of MAs induced by both insecticides in the present study varied between 19% and 29.6% (Tables 1 and 3 and Figure 5a) which was more or less concentration dependent. High concentration of cypermethrin caused significant MAs (p<0.01), while medium and high concentrations of malathion produced significant MAs (p<0.05) compared to the control (0.42%). The same significance pattern was observed on Total Aberrations (TAs) induced by both insecticides. The results indicated that malathion induced genotoxicity aberrations more than cypermethrin where it caused significant aberrations at 1/2 EC (p<0.05) and EC (p<0.01).
Both cypermethrin and malathion induced a wide range of chromosomal aberrations including bridges, vagrants, laggards, and stickiness as well as nuclear aberrations such as multipolar and binucleated cells, micro-, lobed, blebbed and vacuolated nuclei. Cypermethrin caused more bridges and binucleated cells that were concentration dependent, while malathion induced more vagrants especially at low and medium concentration. Anaphase bridges are forms of clastogenic genotoxicity that result from breaks of chromosomes or chromatids and exchanges that result dicentric chromosomes [24] or chromosome segregation errors of fused chromosomes/sister chromatids occurred before mitosis [43]. Lagging chromosomes refer to complete chromosomes that are not integrated into the mitotic spindle during anaphase due to spindle irregularities that lead to unequal separation of nuclei [44].
Both low and high concentrations of cypermethrin and malathion induced interphase micronuclei, which may be formed by lagging chromosomes that appear in mitotic phases and excluded from the nucleus [44, 45]. Binucleated cells and abnormal nuclei are formed when cleavage stage arrest occurs during cytokinesis [46]. These abnormal nuclei usually recognized and removed by physiological apoptosis machinery during normal cell proliferation [47]. Generally, nuclear abnormalities may also be formed during elimination of amplified DNA created during bridges-break induced by material toxicity [22,48].
Nuclear aberrations like blebbed and lobed nuclei are useful markers of genotoxicity [49], while formation of lobed nuclei can indicate cell death process [50]. Micronuclei are the results of acentric fragments, lagging chromosomes, chromosome breakage or whole chromosomes that fail to incorporate into one of the daughter nuclei during telophase of mitotic cells [51,52]. It is suggested that loss of genetic material reveal genomic instability [53] and may reveal cell death [54].
Formation of significant NAs especially at higher concentrations of cypermethrin or malathion confirmed their genotoxic activity. Sticky chromosomes were formed by low concentrations of both insecticides. These sticky chromosomes are sub-chromatid bridges that attach the two chromatids together [44,55] ensuring the highly toxic effects of insecticides. However, occurrence of polyploidy was very scarce in the present study that is comparable to Kocaman and Kilic [40], who observed low frequency of ploidy compared to other aberrations when tested ethephon and cyclanilide insecticides. The results of this study confirmed that cypermethrin causes chromosomal aberrations, which is in agreement with Rao et al., [27], while opposes Asita and Makhalemele [56], who reported cypermethrin (Alpha-thrin) as cytotoxic, but not genotoxic at various concentrations. However, malathion is more genotoxic than cypermethrin. The clastogenic activity of malathion was exhibited as formation of micronuclei, laggards, bridges, binuclei and multinucleated cells, which is in agreement with results on onion [6], bone marrow cells and peripheral blood [7] and A. cepa cells and rat hepatoma tissue culture [8].
Addition of 4 µg/ml peppermint extract to cypermethrin exerted slight modulation on bridges formation, while addition of 8 µg/ml peppermint extract exhibited a considerable reduction of bridges at the low (6.2 to 4.4), medium (8.6 to 5.8) and high concentration (12.6 to 3.4). Peppermint extracts reduced bridges formation at high concentration of malathion (6.0 to 2.6). Addition of 8 µg/ml peppermint extract to cypermethrin or malathion showed remarkable mitigation effect on TAs formation, especially cypermethrin, which reduced TAs at low (1.5 to 1.3%), medium (1.8 to 1.3%) and high concentration (2.8 to 1.6%). Peppermint extract (4 µg/ml) mixed with cypermethrin also reduced aberrations at low concentration (1.5 to 1.0%) and considerably mitigated TAs formation when mixed with low (2.0 to 1.0%) and medium concentration (2.4 to 0.9%) of malathion.
Insecticides are accepted worldwide to protect crops and improve yield. The results from this study clearly revealed the cytotoxic and genotoxic effects of cypermethrin and malathion in the form of reduced MI below 50% of the control and irregularities in cell proliferation kinetics. Cypermethrin and malathion induced significant clastogenic abnormalities. According to results, application of peppermint extract to insecticides modulated its toxic effects and reduced their damage to cells of A. cepa roots. Further studies to illustrate and standardize dose, duration and extraction procedures of peppermint are required.
The authors are grateful to the Deanship of the Scientific Research, King Faisal University, Saudi Arabia for providing financial support for this project (Grant No. 150151).
The authors have no relevant financial or non-financial interests to disclose.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Osman AH El Amin and Hala R Nosiar. The first draft of the manuscript was written by Osman AH El Amin and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ibrahim RIH, Nosiar HR, El Amin OAH. Peppermint (Mentha piperita) extract modulates the genotoxic effects of Cypermethrin and Malathion insecticides on Allium cepa root cells. AGBIR.2023; 39(3):552-559.
Received: 20-Feb-2023, Manuscript No. AGBIR-23-89684; , Pre QC No. AGBIR-23-89684 (PQ); Editor assigned: 22-Feb-2023, Pre QC No. AGBIR-23-89684 (PQ); Reviewed: 07-Mar-2023, QC No. AGBIR-23-89684; Revised: 15-Mar-2023, Manuscript No. AGBIR-23-89684 (R); Published: 10-Apr-2023, DOI: 10.35248/0970-1907.23.39.552-559
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
