Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2023) Volume 39, Issue 1
The non-taxonomic term, actinomycetes are a big group of simple prokaryotic Gram-positive bacteria and represent a group of genera that improve plant growth as well they act as biocontrol agents for many plant pathogens. These filamentous bacteria secrete many secondary metabolites which act as plant growth promoters or alternative agents to different used fungicide. The aim of this study was isolation and molecular characterization of some plant growth promoting actinomycetes from cultivated soil. In this study, out of twenty actinomycete isolates obtained from soil on starch nitrate agar, 5 bacteria isolates showed highly growth on chitin agar and were highly producers for antimicrobial agents against Escherichia coli and/or Fusarium oxisporium. All these isolates were characterized and identified using morphological, physiological and molecular methods. These isolates were screened for Indole Acetic Acid (IAA) production which is important in promotion of plant growth. The detected quantities were ranged from 1.2-3.1 mg/l. the most active isolate was isolate SHR13, thus it was selected for further investigation. The antagonism activity of this isolate against some bacterial and fungal pathogens was evaluated. Efficient antagonism activities were recorded against F. oxysporum and Aspergillus niger while the highest antibacterial activity in vitro was evaluated against Klebsiella pneumoniae, Pseudomonas, Acinetobacter and E. coli. The obtained inhibition zone diameter was ranged from 151-25 mm. The percentage of fungal growth inhibition by the selected isolate SHR513 in the broth medium (in vitro inhibition) was up to 94% which cause hyphal segregation which may due to secondary compounds with fungicidal properties. In conclusion, the results obtained highlight the efficacy of the selected bacteria strain SGR13 as biocontrol agents for broad range of plant pathogens and can be utilized as biocontrol agent for some fungal pathogens in plant experiments.
Chitinase; Antifungal; Fusarium; Streptomyces; Indole acetic acid
Isolating microbes from diverse natural ecological units has led to new metabolites with structural diversity. Soil of Saudi Arabia have been less explored as ecological sites for the discovery of new bioactive compounds from soil microorganisms or from thermophilic bacteria which flourish at high temperatures and produce many important enzymes and metabolites, used to control different plant pathogens [1-3]. There are many plant diseases limited the sustainable development of many plants, thus efficient biological control of these pathogens by eco-friendly and active agents considered opportunities[4]. Certain fungi especially Fusarium can stay for more than 10 years as dormant spores in the rhizosphere soil or on plants until they found their hosts where they proliferate inside the host and act as a source of new infection by the dispersal of fungal spores by wind, water, soil or insects [5]. The most dangerous plant diseases are plant wilt, leaf spot, anthracnose, rust, blight, scab, gall, damping off, rot of root and powdery mildew and fungal infection of leaves may harmfully affect the whole plant, which decrease crop production and quality, thus management process to biocontrol and prevent the spread and progress of these diseases have a good impacts on crop yield [6-8]. The virulence, incidence, and severity of many pathogens biocontrol management strategies depend on special types of microorganisms that can be adopted to remove or eradicate plant diseases are well studied [9].
Soil actinomycetes had many biotechnological applications in agriculture, medicine and food sectors. For rapidly growing world population and to maintain a healthy environment, it is of essence to biocontrol of dangerous plant fungal diseases to increase the production and quality of food, feed and fiber. Over the past centuries, the use of chemical based pesticides and/or fertilizers is not recommended due to the unexpected hazards to the environmental and residual accumulation in human bodies [10]. Stringent laws and regulations must be applied to prevent the use of agrochemicals which are on degradable or unsafe. Nowadays, most of the agriculture depends on natural microorganisms instead of agrochemicals like fertilizer and pesticides to improving crop quality and quantity, thus there is a change in attitude of people towards growth promoting actinomycetes and many scientists diverted their attention towards the alternative natural agents which led to the development of bioagents and antagonistic microbes [11].
Actinobacteria are widely distributed in biotic sources like soil and water and live as saprophyte microbes, capable of decomposing lignin, chitin, pectin [12]. The induced chitinase produced from actinomycetes in the growth medium has the greatest ability for chitin hydrolysis which had pivotal roles in the bioconversion of chitin in the fungal cell walls, exoskeleton of insects and chitin wastes to soluble materials [13,14]. Numerous plant fungal diseases can be controlled by antagonistic Actinobacteria which poses eco-friendly relationship with plants due to the production of anti-pathogenic agents, competition for space and nutrients and/or enhancing the host defensive mechanism [15,16]. A number of bacteria have been identified as possible biocontrol agents due to the large number of secondary metabolites and the most important antagonistic bacteria belong to the genera of Streptomyces, Bacillus, Hypericum and Pseudomonas [17-19]. Also, some genera of fungi like Ulocladium and Trichoderma have the ability to control various bacterial and fungal diseases. The use of antagonistically important Streptomyces is rapidly increased due to the unique ability to produce secondary volatile or non-volatile antimicrobial substances with broad spectrum biocontrol activity. Especially, most of these secondary metabolites can be used to control plant diseases [4] and have significant roles in the promotion of plant growth, establishment of plant defense means and induction of plant systemic resistance [10,11]. Verma et al., [20] isolated three Streptomyces species which promote plant growth regulators and inhibiting the growth of Alternaria. Also, Streptomyces sp. H3-2 inhibit the growth of seven phytopathogenic fungi in vitro with EC50 value of 8.83 µg/ml, while higher EC50 values were recorded by Jing et al., [21].
Recently, different kind of compounds with antimicrobial activity was extracted using different solvents like methanol and ethyl acetate. Extracts of Streptomyces sp. showed more effective antifungal activity [5] which inhibit fungal growth and spores germination and the treated colonial tops of F. oxysporum became swelling and the mycelia had abnormal morphology which may due to extensive cell wall degradation, membrane denaturation and disappearance of some cellular contents [22,23]. Analysis of Streptomyces extracts showed two phenolic compounds with antimicrobial agents [24]. Phenolic compounds, ester, alkane, and other hydrocarbon materials, extracted from bacteria had excellent inhibitory effect on many bacterial pathogens like S. aureus, E. coli, and S. mutans [25-27]. Also, Streptomyces sp. produced many compounds with strong biocontrol activity against Fusarium during fermentation which contained plant growth promoted agents or siderophores. This study aimed to the selection of some Actinobacteria with biocontrol activities against some phytopathogens.
The fungal isolates were from the microbial culture collection, Faculty of Science, Jeddah, Saudi Arabia while the pathogenic bacterial isolates were obtained from King Fahd General Hospital, Jeddah City. The blood agar culture medium was used for growth of some pathogenic bacteria whereas Sabouraud Dextrose agar medium was used for fungal growth.
Chitin and colloidal chitin preparation
The exoskeletons of the shrimps were collected from Jeddah fish market, Kingdom of Saudi Arabia, washed several times, dried and at room temperature, cut to pieces and dried. Approximately 500 g of the dried shrimp shells were de-mineralized for 24 hr at room temperature in 5% HCl (v/v), washed with water and de-proteinized by 3% NaOH solution at 98°C for 4 hr. The obtained crude chitin was washed with water, dried at 50°C, powdered, sieved through 300 mesh sizes and was used to prepare colloidal chitin and chitin agar medium [28].
Sample's collection and bacteria isolation: The present investigation was carried out to isolate and identify filamentous bacteria from different soil samples collected from the rhizosphere region of some grown plants at two farms at Jeddah and Yanbu Al-Nakheel, Western region, Saudi Arabia. Randomly, ten different cultivated soil samples of 100 g each and 10 cm depth were collected in sterile plastic bags, dried and sieved. Actinomycetes isolation was carried out on plates of starch nitrate agar [29] and the inoculated plates were incubated for 5 days at 30°C. Actinomycete colonies were selected, purified and preserved on Starch nitrate agar medium at 4°C until used. All isolates were screened for antagonistic activity, growth on chitin agar and IAA production.
Degradation of chitin on the solid medium: The All bacterial isolates were screened for chitinases production on Mineral chitin agar medium [30]. All inoculated plates were incubated 5 days at 30°C and the presence of clear zone around the growth, confirm positive results. The best growing bacteria were selected, named SHJ5, SHJ10, SHG13, SHG15 and SHG20 and their possible abilities to produce chitinase was confirmed in mineral chitin broth medium.
Preculture preparation and growth in liquid medium: Each tested isolate was grown in 50 ml of starch nitrate broth medium in 250 ml Erlenmeyer flasks, incubated at 30°C and 100 rpm in orbital shaker incubator for 2 days. After that, 2 ml (4 × 106 CFU/ ml) of the bacterial inoculums was used to inoculate each Erlenmeyer flask (capacity 250 ml) containing 50 of the fresh sterile mineral chitin broth medium and all flasks were incubated using shaking incubator (100 rpm and 30°C for 5 days). The cells were collected and the filtrate was used to measure chitinase production [31].
Chitinase assay: Chitinase activity was determined spectrophotometrically by estimating the amount of free reducing groups formed after colloidal chitin hydrolysis as described by Trachuk et al., [28]. The reaction mixture was composed of 0.5 ml of 1% colloidal chitin suspended in 0.1 M sodium acetate buffer (pH 5.5) and 0.3 ml of enzyme solution. After 10 min of incubation at 37°C, 0.75 ml of dinitrosalicylic acid reagent (10 g NaOH, 10 g 3, 5 dinitrosalicylic acid, 2 g phenol, 0.5 g sodium sulfite and 200 g potassium sodium tartrate per 500 ml) was added to stop the reaction. The mixture was heated in a water bath for 10 min at 100°C, centrifuged at 8000 rpm at 4°C for 10 min and its absorbance was measured at 530 nm. N-acetylglucosamine standard curve was prepared and enzyme activity (U/ml) was calculated. Unit is the amount of enzyme that produces 1 μM of N-acetylglucosamine per a min at 37°C [28].
Quantification of plant growth regulators and phosphate solubilization by the selected bacterial isolate
All isolates were screened for IAA production in medium supplemented with 2 mg/ml of L-tryptophan at a pH of 7.0. After growth, the filtered sterile supernatant was used for IAA extraction with ethyl acetate [32] and the quantity was recorded by measuring the absorbance at 530 nm [33] and the quantity of IAA produced by each bacterium was estimated from standard curve of IAA. Similarly, the amount of Gibberellic acid (GA3) produced was estimated from a standard curve prepared using gibberellic acid according to Holbrook et al., [34] and Ashkan et al., [35]. Also, the selected bacterial isolates were screened for phosphate solubilization using Pikovskaya’s medium which contained tricalcium phosphate and the mean diameter of the clear zone (mm) around the colony was measured [36].
Enzyme assay of ACC deaminase
From a stock solution of α-ketobutyrate (Sigma-Aldrich Co., Mumbai, India), different dilution ranging between 0.1 and 1.0 nmol in Tris–HCl was prepared. To 200 μl of each dilution, 300 μl reagent (0.2 % of 2,4-dinitrophenyl hydrazine, pH 8.5) was added and the color was developed by the addition of 2 ml of 2 M NaOH. ACC deaminase activity was measured in bacterial cells after growth minimal medium (g/l: 4 NaPO4, 0.6 NaH2PO4, 0.2 MgSO4.7H2O, FeSO4, 2 gluconic acid, 2.0 glucose and 2.0 citric acid, 1 ml trace elements, pH 7.2) with 1-AminoCyclopropane-1-Carboxylic acid (ACC) as a nitrogen source for 2 days at 100 rpm at 30°C. The cell was collected and 30 µl of toluene were added to the cell suspension and 100 µl aliquot of the toluenized cells was used to determine ACC deaminase activity at 540 nm [37].
Identification of the isolates
The most active bacterial isolates were selected and identified to genus level initially by morphological and physiological tests in addition to 16SrRNA [31,38]. The 5 selected actinomycete isolates, SGJ5, SHJ10, SHR13, SHR15 and SHR20 were characterized using many morphological, physiological and biochemical tests after incubation at 30°C for 7 days on starch nitrate agar medium. Preliminary observations and tests including colony color, the aerial and substrate mycelia morphology, spore chain shape and morphology and other cultural characteristics like mycelium diameter and diffusible pigment production were detected using oil immersion lens of the light microscope (Olympus, Optical. Co. Ltd, Japan) as recommended by William et al. The bacterial isolates were biochemically characterized by Gram stain, starch hydrolysis, catalase, oxidase, and melanin pigment production, resistant to different antibiotics, temperature and pH ranges and NaCl tolerance. Identification was carried out according Hoischen et al., [39] and Chukwuneme et al., [40].
Molecular characterization of the selected isolate
Molecular characterization of the bacterial isolates using 16S rDNA sequencing is particularly important in the case of bacteria to determine phenotypic profiles and it is the correct way to identify bacteria. Also the proportion of guanine and cytosine (constant) was determined for each isolate. Each bacterial isolate was grown in starch nitrate broth medium in a rotary shaker at 30°C for 2 days at 100 rpm. Cells were collected, and DNA was extracted using QIAamp DNA Mini Kit and amplified in a 100 µl reaction using two primers, designed based on the highly conserved region of 16S rDNA from various bacteria [41] and the amplified PCR part was sequenced using big dye terminator cycle sequence kit. The sequence of the DNA was determined and compared to the GenBank database using the BLAST program.
Preparation of bacterial filtrate for antagonistic activity
The antagonistic activities of the tested bacteria against pathogenic microbes was used for testing the antimicrobial activities of the cell free culture media of the actinomycetes on the growth of the tested pathogenic microbes. To prepare the cell free culture medium, the tested bacteria were grown in starch nitrate broth medium for 5 days at 100 rpm and 30°C. Growth of bacteria was measured by determining the optical density at 550 nm. The bacterial cells were collected by centrifugation at 5000 rpm for 10 min and the bacterial filtrate was collected and filter sterilized (Milipore filter, 0.45 μm) and the sterile filtrate was used to detect its effect on fungal and bacterial growth.
Activity of the tested bacterium against some bacterial pathogen
The antimicrobial activities of the bacterial isolate were detected using agar well diffusion assay against some human pathogenic bacteria (Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter aerogenes and Acinetobacter baumannii). Culture of the previous bacteria was obtained from Maternity and Children's Hospital in Jeddah, Saudi Arabia. Presence of the inhibition zone around the well indicated positive results [42].
Antifungal activities of bacterial isolates using dual-plate confrontation assay
The target fungi were cultured on potato dextrose agar (g/l): (fresh potato 200.0, starch 20.0, agar 13.0, pH 7). The antagonistic activity of the bacterial isolates was detected by their abilities to inhibit the growth of F. oxysporum using the modified dual-plate confrontation assays. Also, the antagonistic activity of the bacterium SHG13 was recorded against different fungal pathogens which were obtained from the culture collection of the Microbiological Lab., Faculty of Science, Jeddah, Saudi Arabia. Each pathogenic fungus was grown in the center of the plate and four agar wells were done at the edge of each plate. The sterile supernatant was used to fill each well with 100 µl and the plates were incubated at 25°C for 5 days. The percentage of inhibition was determined from this equation:
The inhibition rate (%)=(Colony diameter of the control – Colony diameter of the tested × 100)/(Control diameter)
Antagonistic activity (dry weight inhibition) in liquid medium: Five fugal growth discs (7 days old) of the tested fungus, F. oxisporium, was grown in 50 ml of Sabouraud Dextrose liquid medium in 250 Erlenmeyer flasks, incubated at 100 rpm for 7 days at 25°C. The culture medium was filtered and fungal dry was recorded after drying at 60°C for 3 days. Also, the pathogenic fungal growth was recorded after treated with different concentrations of the tested bacterial filtrates (0-15 ml/ 100 ml). The dry weight of the pathogenic fungus was determined as mg/l according to the method of Bouknight and Sadoff [43] and the antagonistic effects of the bacterial species was calculated from this equation:
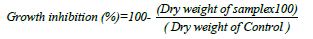
Statistical analysis: Data was statistically analyzed by t-Test to determine the differences between control and treated sample using SPSS software 16 and Two-way ANOVA test was carried out to detect the effect of different factors, P<0.05 are considered significant.
Actinomycetes were isolated from soil collected from two different farms on starch nitrate agar medium. The different isolates were selected, purified and screened for IAA production and any antagonistic activities against two tested pathogens, F. oxisporium and E. coli. Out of twenty actinomycete isolates obtained from soil on starch nitrate agar, 5 bacteria isolates, SHJ 5, SHJ10, SHR13, SHR15 and SHR 20 Figure 1 showed highly production of IAA which is important in promotion of plant growth and the detected amount was ranged from 1.2-3.1 mg/l. Their growth, color and source of isolation were summarized in Table 1. These isolates were highly producers for antimicrobial agents against E. coli and/or F. oxisporium. The isolate SHR13 showed the highest antagonistic activities against F. oxisporium while the other isolates showed moderate antagonistic activities. Isolate SHR13 showed excellent activities against E. coli (Inhibition zone diameter was 20.01 mm) while isolate SHJ5 and SHR 20 showed no activities and isolate SHJ10 and SHR15 showed moderate activities where the inhibition zone diameter were 12.32 and 10.01 mm, respectively. Also, the five actinomycete isolates were selected for growth on chitin agar and chitinolytic index was calculated for each isolate. All the tested bacterial isolates grow on chitin agar medium, the growth of the isolate SHR13 was high while the growth of the other isolates were moderate. The chitinolytic index was calculated for each isolate of the selected actinomycetes and it ranged from 1.0 for the isolate SHJ5 to 2.2 for the isolate SHR13. Growth and chitinase production (u/ml) in liquid medium were detected for the selected actinomycete isolates. The highest growth and chitinase production (3.24 u/ml) was recorded for isolate SHR13. The numbers of unites were determined from a standard curve of N- acetyl glucosamine (Tables 1 and 2).
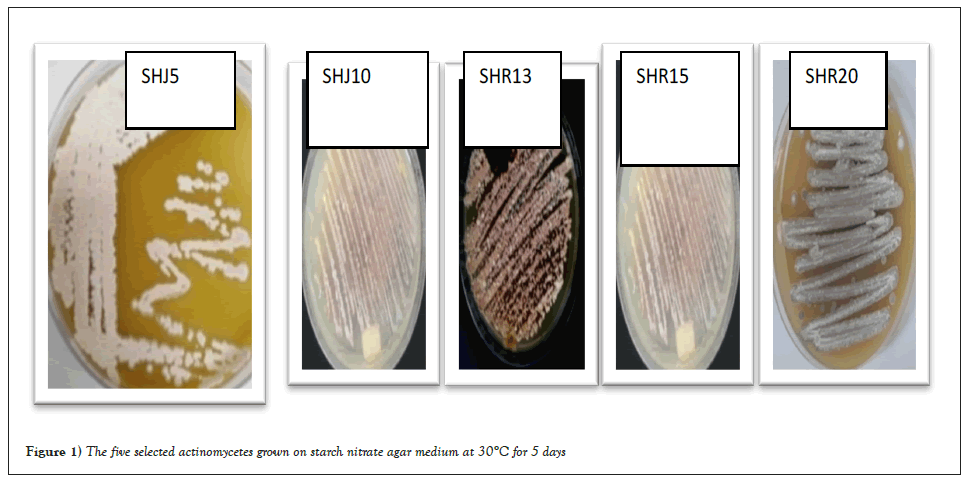
Figure 1: The five selected actinomycetes grown on starch nitrate agar medium at 30°C for 5 days.
| Bacterial isolate | Source | Color | Starch nitrate agar | IAA (mg/l)# | Antagonistic activity | |
|---|---|---|---|---|---|---|
| F. oxisporium | E. coli** | |||||
| SHJ5 | Jeddah | White | ++ | 2.2 ± 0.05 | M | ND |
| SHJ10 | Jeddah | Pink | ++ | 2.3 ± 0.02 | M | 12.32 ± 2.2 |
| SHR13 | Yanbu | Pink | ++ | 3.1 ± 0.55 | H | 20.01 ± 2.1 |
| SHR15 | Yanbu | Pink | ++ | 1.64 ± 0.15 | M | 10.04 ± 0.3 |
| SHR20 | Yanbu | Gray | ++ | 1.29 ± 0.41 | M | ND |
Note: ++: high growth, M: Moderate (less than 50%), H: High (more than 50%), **: Diameter of inhibition zone (mm) ± standard deviation, ND: Not detected, #: Concentration of IAA in medium with 2% tryptophan.
Table 1: The source of the isolated bacteria, their color and growth on starch nitrate agar and production Indole Acetic Acid (IAA) in addition to the antagonistic activity against Fusarium oxisporium and E. coli.
| Bacterial isolate | Detection on solid medium | Detection in Liquid medium | |||
|---|---|---|---|---|---|
| Growth on Chitin agar | Chitinolytic index | Growth A550nm | chitinase activity | ||
| A530nm | (u /ml) | ||||
| Control** | + | 1.4 | 1.24 ± 0.158 | 1.48 ± 0.106 | 1.99 |
| SHR5 | + | 1.0* | 1.08 ± 0 .121* | 1.14 ± 0.015 | 2.26 |
| SHR10 | + | 1.7* | 1.05 ± 0.144* | 1.11 ± 0.031 | 2.21 |
| SHR13 | ++ | 2.2* | 1.29 ± 0.158 | 1.60 ± 0.030 | 3.24* |
| SHR15 | + | 1.1* | 1.08 ± 0.108* | 1.22 ± 0.039 | 2.42 |
| SHR20 | + | 1.2* | 1.21 ± 0.109 | 1.11 ± 0.012 | 2.18 |
Note: Control: Bacillus sp., ++: high growth, +: moderate growth, *The difference is significantly at P ≤ 0.05 compared to control.
Table 2: Growth on chitin agar and chitinolytic index for the selected actinomycetes and production of chitinase (u/ml) in liquid medium.
The selected actinomycetes were examined and morphologically characterized. All isolate were Gram positive filamentous bacteria with high growth on ISP1 medium and had well developed aerial and substrate mycelia. The diffusible pigment was obtained from isolate SHR13 and SHR15 (Table 3). Melanin pigment was produced on IPS7 only by isolate SHJ5 and SHR20 while Hemolysis on Blood Agar was negative (γ type) for all isolates. Gelatin and starch hydrolysis, cellulase and protease production, the use of citrate and reduction of nitrate were positive for all isolates. Growth in the presence of NaCl (%) and temperature (°C) and pH ranges were summarized in Table 4. Antibiotic susceptibility pattern of the tested bacterial isolates to some antibiotics were summarized in Table 5.
| Isolate no. | Gram stain | Shape | Growth on ISP1 medium | Color of aerial mycelium | Color of substrate mycelium | Soluble pigment |
|---|---|---|---|---|---|---|
| SHR5 | G+ve | Filamentous | Heavy | White | Dark yellow | - |
| SHR10 | G+ve | Filamentous | moderate | Pale yellow | Brown | - |
| SHR13 | G+ve | Filamentous | Heavy | Pale pink | Red | + |
| SHR15 | G+ve | Filamentous | Heavy | Pink | Brown | + |
| SHR20 | G+ve | Filamentous | Heavy | Gray | Dark gray | - |
Note: G+ve: Gram positive, +: Positive result; -: Negative result.
Table 3: Morphological characters of the 5 selected actinomycete isolates
| Test | SHR5 | SHR10 | SHR13 | SHR15 | SHR20 |
|---|---|---|---|---|---|
| Melanin pigment on IPS-7 | + | - | - | - | + |
| Starch hydrolysis | + | + | + | + | + |
| Acid production | + | + | + | + | + |
| Growth in the presence of NaCl (%) | 3-10% | 3-10% | 3-5% | 3-12% | 3-10% |
| Temperature range (◦C) | 20-45 | 20-50 | 20-45 | 20-50 | 20-45 |
| pH range | 5-8 | 5-9 | 5-9 | 5-8 | 5-9 |
| Gelatin hydrolysis | + | + | + | + | + |
| Use of citrate | + | + | + | + | + |
| Nitrate reduction | + | + | + | + | + |
| Protease production | + | + | + | + | + |
| Cellulase production | - | + | + | + | |
| Hemolysis on Blood Agar | γ | γ | γ | γ | γ |
Note: +: Positive result; -: Negative result.
Table 4: Phisiological characters of the 5 selected actinomycete isolates.
| Isolate no. | *Resistance to antibiotics | ||||||
|---|---|---|---|---|---|---|---|
| AK | CAZ | PRL | IMI | ATM | CIP | ||
| SHR5 | S | R | R | S | R | S | |
| SHR10 | S | R | R | S | R | S | |
| SHR13 | S | R | R | S | R | S | |
| SHR15 | S | R | S | S | R | S | |
| SHR20 | S | R | R | S | R | S | |
Note: *(AK): Amikacin; (CAZ): Ceftazidime; (PRL): Piperacillin; (IMI): Imipenem; (ATM): Aztreonam; (CIP): Ciprofloxacin, R: Resistant (16 mm or less); S: Sensitive (20 mm or more).
Table 5: Antibiotic susceptibility of the tested bacterial isolates to some used antibiotics.
Figures 2a-2b showed the growth of the isolate SHR13 on chitin agar, Gram stain and resistance to some antibiotics in Tables 4 and 5).
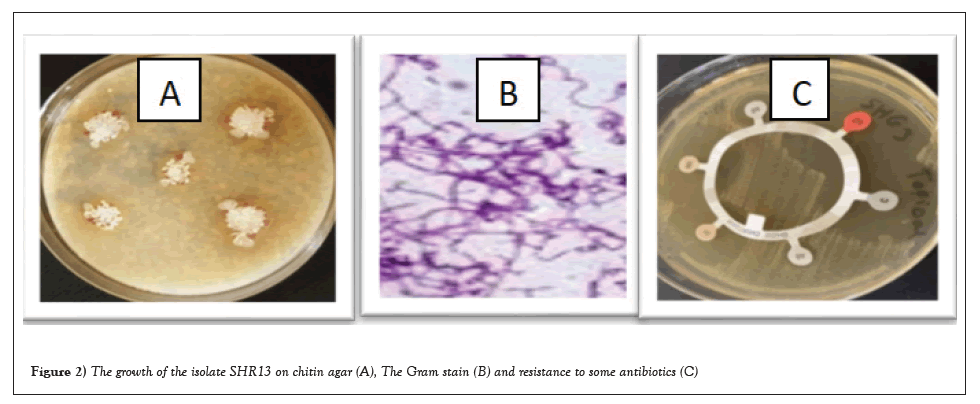
Figure 2: The growth of the isolate SHR13 on chitin agar (A), The Gram stain (B) and resistance to some antibiotics (C).
Also, DNA was extracted from the five bacterial isolates, purified, amplified and sequenced. The obtained data were compared with the found at the GenBank data base and the phylogenic tree was obtained. The bacterial isolates were identified as S. daquingensis NEAU-ZIC8, S. griseocameus DSM4004 S. abyssalis YIM10400, S. thermodiastticus JCM4840 and S. hebeiensis YIM001 (Figure 3 and Table 6). There are four different bases and the frequency of the four nitrogenous base attached to the sugar can vary between nucleotides. The bases on each strand pair up with each other, holding the two strands of DNA in a double helix. The bases always pair up in the same way and the tested actinomycetes are rich with cytosine and guanine and their percentage were more than 60% (Figure 4).
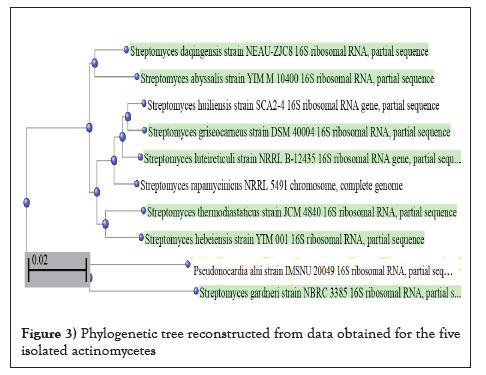
Figure 3: Phylogenetic tree reconstructed from data obtained for the five isolated actinomycetes.
| Isolate no. | Species | Identity (%) |
|---|---|---|
| SHR5 | Streptomyces daquingensis NEAU-ZIC8 | 99 |
| SHR10 | Streptomyces griseocameus DSM4004 | 99 |
| SHR13 | Streptomyces abyssalis YIM10400 | 97 |
| SHR15 | Streptomyces thermodiastticus JCM4840 | 97 |
| SHR20 | Streptomyces hebeiensis YIM001 | 98 |
Table 6: Results of NCBI BLAST query for the 5 sequences of the selected actinomycetes isolated from soil.
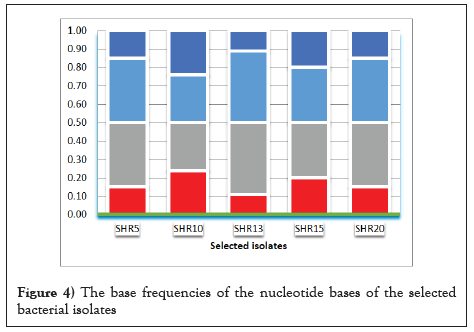
Figure 4: The base frequencies of the nucleotide bases of the selected bacterial isolates.
From the previous results, the isolate SHR13 was the most antagonistic isolate against F. oxusporium and E. coli in addition it was high producer of IAA. The antagonistic activity of this isolate against different pathogens was recorded in Table 7 and Figures 5a-5f. The selected isolate SHR13 was identified as S. abyssalis YIM10400 with 97% similarity level. It had excellent antagonistic activity against some bacterial and fungal pathogens. Efficient antagonism activities were recorded against both F. oxysporum and Aspergillus niger while the highest antibacterial activity in vitro was evaluated against Klebsiella pneumoniae, Pseudomonas, Acinetobacter and E. coli and the lowest inhibition was against Staphylococcus aureus. The inhibition zone diameter was ranged from 11-25 mm.
| Tested Bacteria | Diameter of the inhibition zone (mm) | Tested fungi | Percentage of Inhibition (%) |
|---|---|---|---|
| Staphylococcus aureus | 11.9 ± 1.12* | Fusarium oxysporium (Control) | 60.9 ± 11.03 |
| Enterobacter aerogenes | 20.0 ± 2.19* | Fusarium redolense | 39.0 ± 8.07# |
| Acinetobacter baumannii | 20.7 ± 2.41* | Curvularia khuzestanica | 33.9 ± 7.05# |
| Escherichia coli | 20.1 ± 2.11* | Rhizoctonia solani | 45.9 ± 1.09# |
| Klebsiella pneumoniae | 25.0 ± 2.19* | Aspergillus niger | 55.0 ± 4.11 |
| Pseudomonas aeruginosa | 21.4 ± 6.18* | Candida albicans | 40.0 ± 1.40# |
Note: *: significant results compared to control (5 µg/ disc ampicillin), #: significant results compared to Fusarium oxysporium.
Table 7: The antagonistic activity (diameter of the inhibition zone) and percentage of inhibition (%) of S. abyssalis SHR13 against some pathogenic bacteria and fungi.
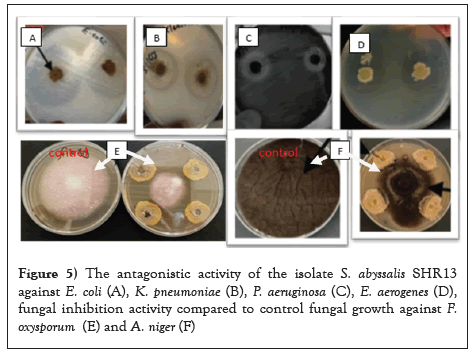
Figure 5: The antagonistic activity of the isolate S. abyssalis SHR13 against E. coli (A), K. pneumoniae (B), P. aeruginosa (C), E. aerogenes (D), fungal inhibition activity compared to control fungal growth against F. oxysporum (E) and A. niger (F).
The filtrate of the isolate S. abyssalis SHR13 was active against all the tested bacteria with inhibition zone diameter ranged from 111.9-25.0 mm. the highest activity was against Klebsiella pneumoniae while moderate activity was against Acinetobacter baumannii, Enterobacter aerogenes, E. coli and Pseudomonas aeruginosa but weak activity was recorded against Staphylococcus aureus (Figure 5a and Table 7). Moreover, the antifungal activity of S. abyssalis SHR13 was detected against different plant pathogens and the percentage of inhibition was ranged from 40- 61%. The highest antifungal activity was against F. oxysporium and A. niger but the lowest antifungal activity was against Candida albicans.
The antimicrobial activities of the isolate S. abyssalis SHR13 against F. oxysporium was determined in liquid medium and fungal dry weight after 5 days of growth was determined (Table 8). The effect of different concentrations of cell free bacterial supernatant on fungal growth were recorded and the results revealed that the pathogenic fungus, F. oxysporium showed higher sensitivity to the bacterial filtrates. Based on the above mentioned results, the inhibition percentage was increased with increasing the used amount of the bacterial filtrate and maximum growth inhibition (93%) was recorded by addition of 20 ml of the bacterial filtrate which may contain antibiotic like materials. Also the isolate S. abyssalis SHR13 showed excellent activity in phosphate solubilization and production of siderphore, ACC deaminase, IAA and GA3 in vitro (Table 9).
| Filtrate Conc. (%) | Dry weight (mg/l) | % Inhibition |
|---|---|---|
| 0.0 (control) | 179 ± 6.9 (100 %) | 0.0 |
| 2 | 168 ± 3.8 (93.8%) | 6.2 |
| 4 | 110 ± 4.6 (61.4%) | 38.6 |
| 8 | 99 ± 6.1 (55.5%) | 44.5 |
| 12 | 69 ± 3.7 (38.55) | 61.5 |
| 16 | 41 ± 2.0 (22.9%) | 87.1 |
| 20 | 11 ± 0.59 (6.1 %) | 93.9 |
Table 8: Effect of different concentrations of the bacterial filtrate of isolate SHR13 (cell free culture medium) after 7 days of incubation on the dry weight (mg) of F. oxysporium and growth inhibition (%).
| Bacterial isolates | Phosphate solubilization (mm) | Siderphore production (mm) | ACC deaminase activity (mmol) | Concentration of IAA (mg/l) | Concentration of GA3 (mg/ml) |
|---|---|---|---|---|---|
| S. abyssalis SHR13 | 6.0 ± 0.91* | 11.4 ± 2.09* | 1.15 ± 0.01 | 0. 44 ± 0.69 | 0.104 ± 0.15 |
Note: *: significant results compared to control.
Table 9: Phosphate solubilization, siderphore production, Indole Acetic Acid (IAA), Gibberellins (GA3) and ACC deaminase production by S. abyssalis SHR13.
Several studies reported filamentous bacteria isolation from soil, extreme environments, and hot deserts and they were of medicinal importance. In recent years, ray or filamentous bacteria (actinomycetes) produce secondary metabolites which considered efficient source of the feasible biocontrol agents to treat fungal diseases of plants than chemically formed fungicides. These bacteria were isolated mainly from soil whereas they can decompose many organic compounds like chitin which is a polymer formed from N-acetyl glucosamine [31]. This complex is mainly found in the cell walls of fungi and exo-skeleton of insects. In this study, 20 gram positive Actinomycetes were isolated and five isolates showed high growth on chitin agar and antagonistic activity against two test pathogens. These isolates were characterized; they have a high G+C base composition, two types of mycelia and chains of conidia. Some morphological features of actinomycetes resample fungi and the most common genus of actinomycetes in soil is Streptomyces which can be used to manage fungal and human pathogens and more than one-half of the medicine used antibiotics like aureomycin, chloromycetin, kanamycin, neomycin, streptomycin, and terramycin produced from soil actinomycetes [44].
The soil-borne Fusarium caused mainly a wilt disease which cause great loss in many plants [45,46]. Soil is the natural habitat for fungi which infect more than 80% of plants and physical and chemical methods are not effective in controlling the spread Fusarium and fungal disease due to the resistant development by the fungus [47]. The secondary metabolites of some functional microbes can be used in biological control which is economically, eco- friendly and safe [48]. In the environment, these bi-agents can degrade chitin which is the second most abundant linear polymer which is a complex of β-1, 4 N- acetylglucosamine, found mainly in fungal cell walls [49]. Several Streptomyces species like S. lividans, S. virdificans and S. halstedii produce chitinase which are used widely in agricultural, biological and environmental process [50-52]. Thus, this study aimed to isolate, identify and characterize soil actinomycetes which produce both chitinase and good antifungal agents against different fungal pathogens without any toxicity to soil. Hemolysis is the breakdown of red blood cells and based on haemolytic activity of the tested bacterial isolate, no hemolysis was recorded, thus, they can be used safely in soil. It was reported that, Actinobacteria had biosynthetic potential to produce large amounts of bioactive secondary metabolites with novel structure and remarkable biological activity in agriculture [53]. Streptomyces sp. ACT7 showed a high inhibitory activity against Alternaria and F. oxysporum and many antifungal agents are produced from Streptomyces like Mildiomycin, which is well known potent fungicide, obtained from S. rimofaciens to treat powdery mildews on cucumber [54,55]. Also, during strawberry fruit storage, Streptomyces was used for its strong inhibitory activity against Colletotrichum fragariae [56]. However, the use of those functional microbes for controlling plant diseases is limited due to commercial growth difficulties in the lab. Conditions or low activity after application, thus isolation and screening for of broad-spectrum and highly efficient antagonistic agents are still necessary [4,21,57]. In this study, five Streptomyces isolates showed inhibition activity against Fusarium and they were identified based on morphological, physiological and biochemical methods and molecular methods according to Williams et al., and Santos-Beneit et al. The phylogenic tree reported that these isolates were different and represented five different species of the genus Streptomyces. It was noted that some bacterial isolates were difficult to be identified by the phenotypic tests, thus molecular techniques are applied for their identification process. Unlike phenotypic assays, gene sequencing is widespread used in the identification of bacteria recovered from different environments which increased dramatically the number of bacterial genera [58]. The ribosomal 16S rRNA, 23S rRNA and Internal Transcribed Sequences (ITS) genes are shared by many of bacterial and fungal species [59]. The highly conserved region of bacteria, the 16S rRNA gene contained the hyper-variable nucleic acid sequences which are targeting by the PCR to detect the conserved nucleic acid sequences can be used to document the bacterial type in the different sources. The hyper-variable sites in the 16S rRNA gene of bacteria is helpful in the bacterial species identification process where nucleic acid are not changed during growth, thus the results of 16SrRNA sequences were collected and compared via electronic database e.g., GenBank [60-63]. At the present, viable test protocols are presented for bacterial identification to the species level using the sequences of 16S rRNA. Similarly, using different characteristics, Streptomyces sp. H3-2 from the rhizosphere of banana was identified and it produce broad-spectrum antifungal agent which affect Fusarium growth, mycelia morphology and spore germination in addition it had biocontrol ability in a pot experiment [23].
In this study, the selected isolate S. abyssalis SHR13 produced chitinase which played a crucial role in fungal growth inhibition and degradation of complex nutrients present in soil. As fungal cell wall structures largely contain chitin, chitinase produced by actinobacteria can be deleterious to fungal pathogens which showed different responses to chitinase from S. griseus due to cell wall degradation [64,65]. The scanning electron microscopic study showed that extracellular enzymes of Streptomyces sp. ACT7 like chitinase degrade the cell walls of F. oxysporum and Alternaria sp. [66].
In this study, the isolate SHR13 was identified as S. abyssalis and had excellent antagonistic activity against the tested fungi and bacteria. It has a good ability to phosphate solubilization and production of siderphore, ACC deaminase, IAA and GA3. Similarly, IAA, GA3 and ACC deamiase were detected in the culture filtrate of Streptomyces sp. SA5 [67]. Production of IAA in growth media of actinomycetes during the metabolism of the amino acid L-tryptophan was confirmed by many authors [68,69] while soil bacteria used root exudates to form IAA. Streptomyces rochei, S. livaceoviridis and S. rimosus from the rhizosphere of tomato, were highly producer of IAA which enhance plant growth [31,70]. The result of this study revealed that the isolate S. abyssalis SHR13 produced ACC deaminase enzyme (EC 4.1.99.4) which had many biological applications, thus interaction of this bacterium with plants, may improve growth and control some pathogens during biotic and abiotic stresses [71-73]. Many bacterial isolates have the ability to produce siderophores, IAA and phosphate dissolving enzymes during their growth which promote plant growth and decrease fungi infection due to compounds that are synthesized by these bacteria [74,75]. Siderophores produced by soil bacteria are of crucial importance, capable of chelating iron and other mineral to make them available for the plants [76-79].
Soil is rich with actinomycetes especially that belong to genus Streptomyces that played excellent roles in soil. They can be isolated and identified using different techniques to study the morphology, physiology and chemistry of the hyphae in addition to molecular methods. Species of genus Streptomyces grow well on mineral chitin agar due to chitinase production that led to fungal cell wall degradation of many fungal diseases. Thus, species of the genus Streptomyces can be used safely to biocontrol many plant phytopathogens and enhance plant growth.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Khallaf H, Amasha R, Alaidaroos B, et al. Isolation and molecular characterization of some plant growth promoting actinomycetes from cultivated soil for management of some pathogenic microbes. AGBIR.2023; 39(1):412-420.
Received: 29-Nov-2022, Manuscript No. AGBIR-22-81628; , Pre QC No. AGBIR-22-81628 (PQ); Editor assigned: 02-Dec-2022, Pre QC No. AGBIR-22-81628 (PQ); Reviewed: 16-Dec-2022, QC No. AGBIR-22-81628; Revised: 26-Dec-2022, Manuscript No. AGBIR-22-81628 (R); Published: 02-Jan-2023, DOI: 10.35248/0970-1907.23.39.412-420
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
