Agricultural and Biological Research
RNI # 24/103/2012-R1
Review Article - (2022) Volume 38, Issue 1
Plant PCD differs genetically and morphologically from the mechanisms of fungi and animals. For instance, classical PCD typically features mitochondrial morphology transition (MMT), condensation of the cytoplasm and its shrinking, detachment of the plasma membrane from the cell wall (in case of fungi), and nuclear condensation. There is now compelling evidence that mitochondria integrate diverse cellular stress signals and initiates the death execution pathway in animals. On the flip-side involvement of mitochondria in regulating PCD in plants is not well known. This review article will help to answer the following questions; how PCD is required for resistance? How PCD and other resistant responses are dependent on each other? How PCD is regulated and is PCDs the same for all pathogens?
Photosynthesis; Stomata conductance; Morphologically; Relative water content
ROS mediated PCD in plant
abiotic and biotic stresses induce the production of free radicals of oxygen (such as superoxide, per hydroxyl radical, and peroxy radical) and hydrogen peroxide (H2O2), and singlet oxygen (1O2) [1]. These ROS molecules pursue damage to macromolecules (DNA, protein, and lipid damage), and also cellular components. ROS are generated from photosystem II activity in the chloroplast, electron transport in mitochondria, b-oxidation in glyoxysome, photorespiration in the peroxisome, and other oxide reduction reaction in the cytosol. The production of ROS in disease resistance response, a process known as oxidative burst, was first observed in potato discs inoculated with a race of Phytophthora infestans that results in a hypersensitive response (HR) [2]. It serves as a secondary signal for programmed cell death (PCD). Higher plants possess various antioxidant enzymes such as superoxide dismutase, peroxidase, and catalase to detoxify these ROS. The early plant response to an attempted infection by microbial pathogens is often accompanied by rapid cell death in and around the initial infection site, a reaction known as the hypersensitive response (HR). While the induction of programmed cell death (PCD) in plants assumes to be a common response to many different types of biotic and abiotic stress. However, plants often trigger a strong resistant response against bio trophic and/or hemi-bio trophic pathogens via cell wall strengthening, ROS production, and PR gene expression etc. Usually associated with ETI not PTI, triggered by a virulence factor. Only occurs in resistant plant/host, not in susceptible plants. While, apoptosis is a type of programmed cell death in animals, the main features in this phenomenon is to cell shrinkage, blabbing of the plasma membrane, condensation and fragmentation of the nucleus, and intra-nucleosome cleavage. Capsizes are cysteine proteases which are found to be involved in apoptosis in animal. Apoptosome which is a wheel shaped protein complex consist with Cyt c, Apsf-1 and procaspase [3].
Plant responses to biotic stress and two-layer immune system
Plants have elaborated and efficient mechanisms to defend the spread of pathogen invasion. In any natural habitat, there is potential continual conflict between pathogenic microbes and larger multi-cellular organisms. For instance; bacteria and fungi, infection of the host provides access to nutrients as well as a site for growth and reproduction.
In response to these pathogens, plants encounter with two main lines of defense. In order to protect host against infection, pathogen (or microbe) associated molecular patterns (PAMPs or MAMPs) are comprehended by the plant and responds to the pathogen. First instance; a recognition receptor (pattern recognition receptors-PRRs; FLS2 and FLS3) of the immune system involved in to identify some conserved molecules (PAMPs) like bacterial flagellin (flg22 and flagII 18), lipopolysaccharide (LPS), peptidoglycan, nucleic acids linked with virus, and double stranded RNA (ds RNA) (Figure 1). Subsequently, PAMP-triggered immunity (PTI) involves expression of defense genes and generation of antimicrobial molecules. When the PAMP system fails to recognize the pathogen as an invader, a second line of plant defense is induced. Plants may produce specific surveillance proteins encoded by resistant (R) genes to recognize effectors (called then avirulenceavr factors and the pathogens being virulent) and mount this second line of defense called effector triggered immunity (ETI). While a good number of pathogens have (race-specific or strain-specific) effectors proteins that counteract to impair molecular components of the plant innate immune response, leading to effectors triggered susceptibility or immunity (ETS) Dinesh.classic hypersensitive response (HR) in Tobacco leaves (Nicotiana tabacum cv. Samsun NN ). Tobacco mosaic virus/N gene interaction: mock treated (left) or inoculated with TMV leads to formation of HR lesions that efficiently restrict the virus to the inoculated regions, in cultivars lacking the N gene, no lesion spots are observed (right) as described by Dinesh [4].
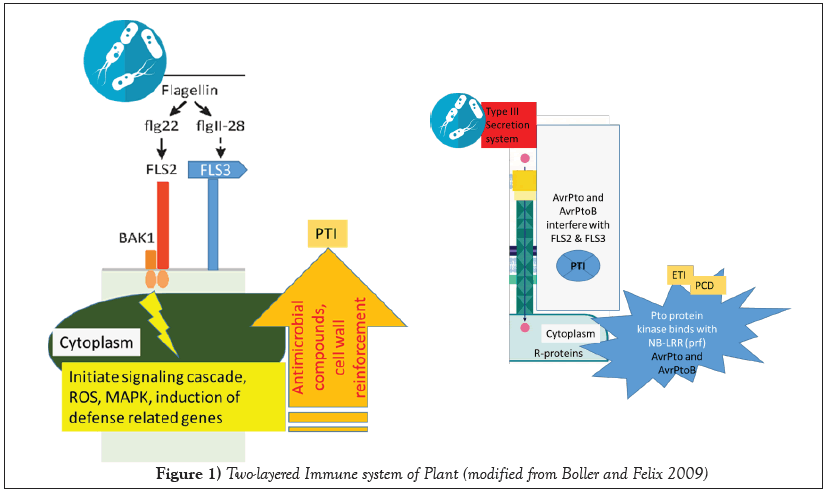
Figure 1: Two-layered Immune system of Plant (modified from Boller and Felix 2009)
Plant cells can response to ETS (i.e pathogen effectors proteins) by a layer of immune responses as known hypersensitive response (HR) [5]. In this context, programmed cell death (PCD) is well notified in relation to the manifestation of the hypersensitive response (HR) caused due to the interactions between a host plant and an incompatible pathogen [6].
In general, the HR reaction involves at the site of pathogen entry and resulting PCD in and around the infection site [7]. It is accompanied by the induction of plant defense responses that serve to confine the pathogen and protect rest of the plant. Figure 2 showed the necrotic lesions characteristic of the HR, associated with the resistance response of tobacco plants to TMV. Especially, in the host-pathogen system, the product of the N gene is required for the recognition of TMV replicate by tobacco cells. By contrast, plants lacking this gene fail to mount HR and instead display widespread cholorosis that is associated with the systemic spread of the virus [8] (Figure 2).
Figure 2: Classic Hypersensitive Response (HR) in Tobacco leaves (Nicotiana tabacum cv. Samsun NN)
Programmed cell death and morphological features
Programmed Cell Death (PCD), is a process of controlled and gene directed destruction of cells and serves a function in defense response to restrict the spread of pathogens but also in the proper development of the multi-cellular body plan [9]. There are several mechanisms by which PCD is executed in plants [10]. For instance; embryo formation, differentiation of treachery elements in water conducting xylem tissues, removal of the aleuronic layer during monocot seed germination, formation of root arenchyma, and epidermal trichomes, another tapetum degeneration, stamen or ovary abortion during male and female flower formation, megaspore abortion, floral organ abscission, pollen self-incompatibility, remodeling of some types of leaf shape, leaf senescence, death of root cell cap, and plant immunity to bio trophic pathogen [11]. Three major forms of PCD can be recognized on the basis of morphological changes: apoptosis, classical plant PCD and autophagy [12]. In animals’ cells apoptosis is defined by rapid changes in mitochondrial morphology (via intrinsic and extrinsic death signals), caused by an up regulation of the mitochondrial division machinery, nuclear and cytoplasmic condensation, and the formation of apoptic bodies containing damaged cellular components [13]. The observed altercation in morphology are involved in the release of mitochondrial proteins (e.g., cytochrome C) into the cytosol, which in turn switched on a range of cellular proteases that carefully degraded the cell and its components. Blocking the change in mitochondrial morphology prevented cell death. It can be concluded that mitochondrial morphology changes are vital to the apoptotic process. While in classical plant PCD typically featured by mitochondrial morphology transition (MMT), shrinking of the plasma membrane from the cell wall and nuclear condensation. By using mitochondrial-targeted green florescent proteins (GFP), Logan speculated that ROS induced changes in mitochondrial morphology (swollen and abnormal appearance) are preceding to PCD in Arabidopsis protoplasts. It has also been coined that mitochondrial morphology transition (MMT) is an early sign of the cell death process in plants. The release of cyst C into the cytosol act as an early event during PCD in cucumber documented by Douce [14]. Similar events were reported for maize suspension cultures cells, protoplasts of tobacco, and suspension cells of Arabidopsis (Figure 3).
Figure 3: Typical cell death pathways: A. Apoptosis pathway in animals, C. Classical plant PCD (Scott and Logan, 2008, modified)
Autophagy is occurring in plants and animals and plays a major role as degradation and recycling system, because it contributes to the turnover of cells by delivering parts of the cytoplasm to lysosomes where they are digested.
Necrotic death is considered as an uncontrolled form of cell death and it results from acute metabolic disruption with ATP depletion, ion deregulations, mitochondrial and cellular swelling and activation of derivative enzymes, all culminating in plasma membrane rupture [15].
Plant mitochondria compartment
Mitochondria are important cell organelles that are highly dynamic, pleomorphic, and comprised of at least six compartments: outer membrane (OMM), inner boundary membrane (IBM), inter membrane space (IMS), crystal membranes (CMs), intracristal space (IS), and matrix (Figure 4). The IBM folds into cristae greatly dispersing its surface area. These cristae contain 80%-90% of the total mitochondrial membrane associated proteins including respiratory chain complexes I-IV, and the ATP synthase complex while most transport system including the adenine nucleotide transporter (ANT), are located in the IBM. In active mitochondria, the protein complexes forming the respiratory chain lead to the generations of an electrochemical proton gradient across the crystal membrane. The reflux of protons into the mitochondrial matrix across the crystal membrane via the ATP synthase complex drives ATP production from ADP and Pi.
Figure 4: The mitochondrion and its permeability transition pore (PTP complex)
The area surrounded by outer mitochondrial membrane (OMM) and inner mitochondrial membrane (IMM) is called inter membrane space. Inside the IMM is called matrix. A diagram of (PTP) complex. The PTP components have not been fully established. Furthermore, the depicted model of protein interactions in the PTP is mostly based on the studies in animals. The main components are VDAC of OMM and ANT of IMM. In addition to them, CypD, which interacts with ANT from the matrix side, and Hxk, which interacts with VDAC from the cytoplasmic side, are involved.
The ATP generated on the matrix side of IMM is then exported in exchange for ADP by the ANT. Mitochondria are not only the major source of ATP but are also involved in various metabolic processes including the biosynthesis of amino acids and nucleotide, vitamins, fatty acids and iron-sulphur clusters. Thus, the organelle plays an essential physiological role in the cell.
OXPHOS system and ROS
Oxidative phosphorylation (OXPHOS) is linked with the electron transport chain in the mitochondria and it is a vital part of metabolism (Figure 5). Electron transport within the mitochondrial electron transfer chain (mETC)- inevitably yields reactive oxygen species such as superoxide anions at the level of complexes I and III of the OXPHOS system which are converted to hydrogen peroxide. Besides, large amount of hydrogen peroxide are produced during biotic stress and leads to the oxidative burst reaction as part of the defense-associated hypersensitive response (HR) resulting cellular PCD [16].
Figure 5: Internal structure of mitochondria highlighting the OXPHOS system
Furthermore, there is increasing evidence that small GTP-binding proteins (RAC/ROP) of plants plays pivotal roles in pathogen induced ROS production through plasma membrane associated NADPH oxidases known as respiratory burst oxidase homologues (RBOHs) [17].
The role of mitochondria in apoptotic cell death
Mammalian mitochondria have been considered as key player in the apoptotic cell death in by opening of a channel that is known as the permeability transition pore (PTP) [18]. The PTP complex is assumed to be formed at the IMM–OMM contact site and is thought to consist of ANT, VDAC, and cyclophilin D (CypD), and other molecules. VDAC involvement in the PTP has been proved by studies using specific anti-VDAC antibodies. It is believed that apoptotic stimuli often influence the opening of PTP, which is accompanied by loss of mitochondrial inner membrane potential, osmotic swelling of the mitochondria, and finally disruption of the OMM. It starts the release of inter membrane space proteins namely cytochrome c, Followed by the assembly of a high molecular weight caspase-activating complex in the cytoplasm [19]. VDAC (30 kDa, polypeptide) is indispensable for PTP opening, hence by blocking VDAC or ANT or by preventing the formation of the PTP the apoptosis pathway hindered as described by Baines [20].
Plant VDACs (voltage dependent anion channels) are encoded by a small gene family
Mitochondrial localization of plant VDAC (a porin type, polypeptide) has been reported in several plant species in pea, potato, tobacco, and Arabidosis. It is believed that VDAC involved in metabolite exchange in the mitochondria and the cytosol. In first instance, rice and Nicotiana tabacum three isoforms of VDAC have so far been reported. While in Lotus japonicas, Medicago truncatula, Arabidopsis thaliana, soybean and each possess five different VDAC isoforms. It is speculated that under certain biotic and abiotic stresses induce the up- regulation of VDAC, therefore, it is likely that enhancing the expression may indeed lead to tolerance against the above stresses.
Caspases which is class of specific cysteine proteases, it specifically recognizes four AAs with Asp (D) at the end (WEHD, LEHD, DEVD, and DEHD). They are produced as inactive and pro-enzymes but activated and cleave some proteins such as DNase inhibitory protein in order for apoptosis to occur. Metacaspases belongs to plant cysteine protease having similar structure and functions. Plant metacaspases have different recognition specificity such as Arg (R) or Lys (K).
VPE; a vacuolar processing enzyme which is protease involves in HR. It induces resistance against TMV in Tobacco, carrying N resistance gene causes HR [21], vpe1 mutation leads to no cell death and more spreading of virus, but does not affects PR1 expression.
VPE1 encodes a protease that may play a role in vacuole collapse (disintegration of vacuole membrane) leading to cell death. VPE is structurally unrelated to caspases but VPE found to be induced in caspase-1 activity. PCD contributes to resistant to virus infection, and PR gene expression is independent of PCD pathway. Hypersensitive cell death is an important resistance response during ETI. PCD can occur independently with pathogen arrest during resistance. Regulation and modulation of PCD is complicated, being affected by salicylic acid, ROS, ion channels, capsizes-like proteins, autophagy, etc.
Citation: Parvin MS, Haque ME. Reactive oxygen species, Programmed Cell Death (PCD) and role of mitochondria in host pathogen interaction. AGBIR. 2022; 38(1):247-251.
Received: 05-Jan-2022, Manuscript No. AGBIR-22-51194; , Pre QC No. AGBIR-22-51194 (PQ); Editor assigned: 07-Jan-2022, Pre QC No. AGBIR-22-51194 (PQ); Reviewed: 21-Jan-2022, QC No. AGBIR-22-51194; Revised: 25-Jan-2022, Manuscript No. AGBIR-22-51194 (R); Published: 07-Jan-2022, DOI: 10.35248/0970-1907.22.37.247-251
