Agricultural and Biological Research
RNI # 24/103/2012-R1
Research - (2023) Volume 39, Issue 6
In the present situation, entire world is looking forward for an alternate energy source due to increased concern on environmental pollution and energy security. In the present aimed at the production of bioethanol by using free and immobilized cellulase from Bacillus sp. In this study, highest cellulase activity was expressed by the bacterial isolate identified as Bacillus subtilis BTSR1 (1.817 ± 0.26 U/mL) after 24 hr incubation at 37°C under static condition. In this study, the extracellular cellulose from B. subtilis BTSR1 strain was produced, purified and estimated to be 46 kDa with specific activity was 302.27 U/mg. Immobilization process was standardized with respect to concentration of CaCl2, sodium alginate and bead size in order to obtain maximum efficiency. Different percentages of sodium alginate gel were tried. However, maximum immobilization efficiency was achieved with 4% alginate with 0.2M CaCl2 and bead size was 10 g. The resultant optimal conditions for the highest CM Case production by Bacillus subtilis under SSF using wheat bran and coffee pulp as substrates were used for the subsequent bioethanol production. Bioethanol production was further optimized by varying substrate concentration (1-5%), Yeast Extract (0.1-0.5%), pH (4-8) and time (2-10 days). Immobilized enzymes produced the highest yield of bioethanol 28 g/l was obtained for 4% of substrate concentration. Immobilized cellulase activity nearly doubled the amount provided from the free enzyme activity. Additionally yeast extract concentration also optimized and ethanol production was found to increase 27 g/l of 0.3% yeast extract concentration. pH and duration were optimized also play a remarkable role in the production of bioethanol. In this study concluded that the maximum amount of bioethanol production was observed in the immobilized enzyme than free enzyme. Thus, immobilized condition was improved the activity of enzyme to produced bioethanol.
Bacillus subtilis; Immobilization; Bioethanol; Cellulase; Alginate
Lignocellulose forms the major structural component of plants. It is made up of cellulose along with hemicelluloses and lignin strongly interlocked by non-covalent and covalent forces. Annual production of lignocellulosic through photosynthesis is 40 billion tones [1]. The problem of increasing the utility of lignocellulose wastes has been known for decades. It is the most dominating waste material from agriculture and forest [2]. Lignocellulosic biomass is a major resource for the production of bio-fuels since it is largely abundant, inexpensive and eco-friendly. Lignocelluloses are employed for traditional applications (paper manufacture, biomass fuels, composting, animal feed, etc.) since long back and novel markets for lignocellulosic have been identified in recent years. In the field of biotechnology, bioconversion of cellulosic biomass to develop novel and useful products is an important area of research.
Cellulose is degraded by a group of enzymes which acts synergistically to randomly cleave the β-1, 4 glucosidic bonds. The enzyme complex responsible for the hydrolysis of cellulose is cellulases. It is an inducible enzyme secreted by wide variety of fungi, bacteria, protozoan, plants and animals. Cellulose is converted into smaller organic compound called cellodextrins or completely into glucose units by the action of cellulases. Three types of synergistic actions are involved in cellulolysis, i) The synergism between endogluconase and cellobiohydrolase (endo-exo synergism), ii) The synergism between two cellobiohydrolase (exo-exo synergism), and iii) The synergism between all the three enzymes of the cellulase complex (endo-exo-β- glucosidase). Celluase are extensively used for the industrial purpose (pulp, textile, paper, food industry, and as an additive in the detergents [3]. Cellulolytic microorganisms can be found in all biota where cellulose biomass accumulates. Cellulase producing bacteria were exploited widely for the extraction, purification and characterization of novel cellulase enzymes, because of their higher growth rate, presence of multi enzyme complex and their wide distribution in different environmental niches. Microorganisms from various environments provides potential source of novel cellulase. Naturally occurring bacterial enzymes from the environment is important in many industrial applications to help overcome costly hurdles.
Members of the Bacillaceae are Gram-positive, endospore forming bacteria which are known to secrete an extensive array of enzymes, including amylases, proteases, β-glucanases, and hemicellulases. Thus far, these organisms have been found to produce only endo-β-1,4-glucanases and have not been capable of extensively degrading crystalline cellulose. The cellulolytic enzymes of Bacillus species are almost entirely extracellular and soluble in form. A variety of species of this genus, including strains of B. subtilis, B. polymyxa, B. licheniformis and B. cereus, secrete cellulases. Hence the present aimed at the production of bioethanol by using free and immobilized cellulase from Bacillus sp.
Isolation of cellulolytic bacteria
The soil samples were collected from dump yards regions of Thanjavur, Tamil Nadu, India (Geographical coordinates are 10.726 N, 79.080 E). Serial dilution technique has been used to isolate cellulolytic bacteria from soil sample about 10-1 to 10-7 dilution factor. The 50 µL of the diluted sample (10-5) was plated on the screening medium composed of Carboxymethyl Cellulose (CMC) containing-1.0% carboxymethyl cellulose, 1.0% peptone, 0.2 % (NH4)2 SO4, 0.2 % K2HPO4, 0.03% MgSO4.7H2O, 0.2% gelatin and 1% agar [4]. The CMC gar plates were incubated for 48 hours at 37°C. For the primary screening of cellulose producing bacteria, point inoculation of the isolates was carried out on the same cellulose screening media plates, followed by incubation at 37°C for 48 hours. The plates were flooded with 0.1% congo red and further washed using 1 M sodium chloride to visualize the zone of clearance [5].
Screening for cellulase enzyme production
The particular strains were further tested for their abilities to produce cellulase under Submerged Fermentation (SMF). The maximum cellulose production isolates were considered for the further study.
Cellulase production
CMC broth CMC 0.5%, Tyrptone 0.20%, KH2PO4 0.4%, MgSO4.7H2O 0.02%., Na2HPO4 0.04%, CaCl2.2H2O 0.0001%, FeSO4.7H2O 0.0004% and pH 7.0] [6] was prepared and allocates 50 ml in 250 ml Erlenmeyer flasks for each of the selected isolated strain. The media were inoculated with 2.5 ml of the selected bacterial isolates from the inoculum media and incubated on 37°C for 48 hours at 150 rpm. After fermentation, the fermented broth was centrifuged to remove unwanted materials at 12000 rpm for 10 minutes at 4°C. Finally, clear supernatant was collected and used to determine enzymatic activity [7].
Partial purification of cellulase
Partial purification was achieved by fractional precipitation using ethanol (50% and 70%). The fractions containing protein pellets were dissolved in 0.01 M phosphate buffer (pH=7), then were examined for cellulase activity and protein content. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE (Laby Instruments Model E-2,)) (8%) for protein molecular weight was estimated according to the standard protocol of Laemmli [8].
Immobilization of enzyme
On calcium alginate beads the basic protocol described by Kierstan and Bucke [9] for immobilization of enzyme on alginate beads was used. Sodium alginate (5 gm) was added slowly to 100 ml of vigorously stirred water. Stirring was continued for 3 hours and thereafter the alginate was left for 24 hours to allow the air bubbles to escape. Next day, 10 ml of buffer A and 2 ml of enzyme was added to the viscous solution of sodium alginate with stirring and the slurry was left overnight to remove air bubbles. The homogeneous slurry was extruded drop wise through a syringe into 0.2 M calcium chloride. Beads were cured in 0.2 M calcium chloride for 4 hours and then washed several times until no protein was detected in the washings. Beads were stored at 4°C in 30 mM calcium chloride solution prior to use.
Bioethanol production
The resultant optimal conditions for the highest CM Case production by Bacillus subtilis under SSF using wheat bran and coffee pulp as substrates were used for the subsequent bioethanol production. The reduced glucose sugar, as the product of lignocellulolytic biomass digestions, was used for fermentation to ethanol by S. cerevisiae. Fermentation media was prepared by mixing pretreated substrate with peptone and yeast extract in 100 ml citrate buffer. Media prepared was sterilized and inoculated with baker’s yeast and 5 ml of free and Na-alginate immobilized enzyme respectively. The fermentation was carried out at 120 rpm and 30°C. The sample was collected at regular intervals of 24 h for the determination of bioethanol. Bioethanol production was further optimized by varying substrate concentration (1%-5%), Yeast Extract (0.1-0.5%), pH (4-8) and time (2-10 days).
Quantitative measuring of bioethanol
Quantitative estimation of ethanol was carried out by the potassium dichromate method [10] where different concentrations (3-18%) of ethanol (99.9%) were prepared giving 5 mL final volume with distilled water. 1 mL of 10% potassium dichromate was added to each concentration and the final volume of each was kept in ice water followed by gently adding concentrated sulfuric acid on the sidewall giving prepared samples for measuring the Optical Density (OD) at k660 nm. The standard graph was plotted and the unknown sample was estimated [11].
Screening of cellulolytic bacteria
Totally 26 cellulolytic bacterial strains were isolated from the soil sample using the 10-5 dilution plate. Among them, six colonies which produced largest zone and hydrolysis the substrate CMC on CMC-Congo red agar plate by cellulolytic bacteria. Bacterial isolates showed different zones of clearance around the colonies. The zone of clearance around the colony is an indicator of cellulolytic potential of the isolates (Figure 1). The primary screening of potential bacterial isolates such as Bacillus subtilis BTSR1, Pseudomonas aeruginosa BTSR2, Serratia fonticola BTSR3, Escherichia coli BTSR4, Clostridium butyricum BTSR5 and Staphylococcus aureus BTSR6 showed maximum zones like 3.68 ± 0.89 mm, 2.84 ± 0.15 mm, 1.73 ± 0.27 mm, 2.75 ± 0.24 mm, 1.12 ± 0.02 mm and 2.97 ± 0.27 mm respectively (Table 1). Utilization of CMC as carbon source is the best for microbial cellulase production [12]. CMC acts as a good carbon source for the production of cellulase production was also reported by Das et al., [13]. Some investigators showed that additional of cellulose, filter paper, CMC, starch or cellobiose to the fermentation medium forwarded cellulase production of cellulolytic organisms such as Cellulomonas sp, Clostridium and Bacillus sp.
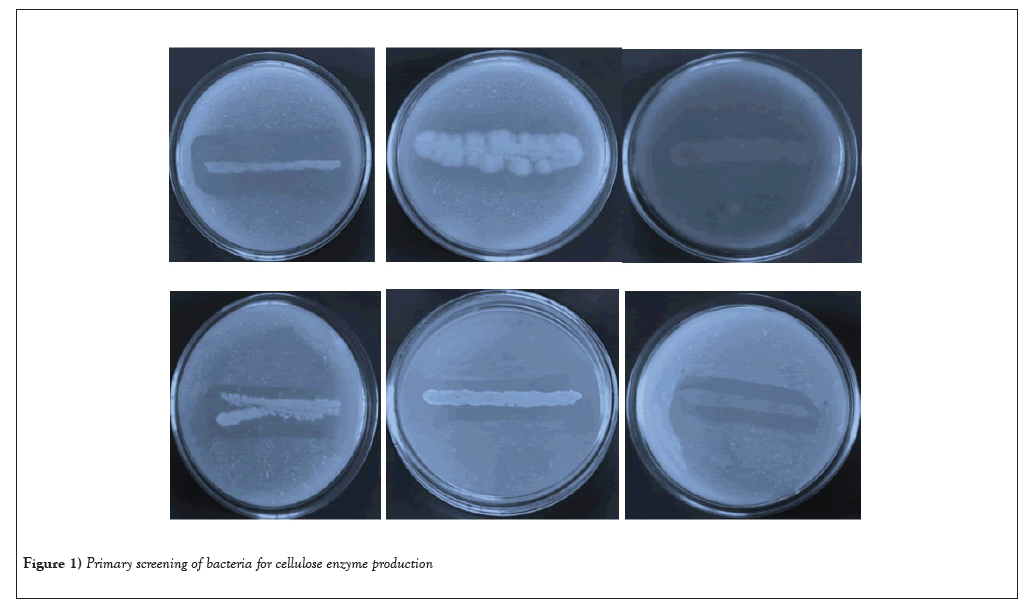
Figure 1: Primary screening of bacteria for cellulose enzyme production.
| S. No | Bacterial isolates | Cellulolytic activity |
|---|---|---|
| Zone of measurement (mm) | ||
| 1 | BTSR1 | 3.68 ± 0.89 |
| 2 | BTSR2 | 2.84 ± 0.15 |
| 3 | BTSR3 | 1.73 ± 0.27 |
| 4 | BTSR4 | 2.75 ± 0.24 |
| 5 | BTSR5 | 1.12 ± 0.02 |
| 6 | BTSR6 | 2.97 ± 0.27 |
Table 1: Primary screening of potential cellulolytic bacterial isolates.
Cellulase production
Bacterial isolates were able to produce cellulose under submerged fermentation. The enzyme activity was measured from CMC agar plates. The cellulase yield was recorded by the cellulolytic bacterial isolate such as BTSR1 (1.817 ± 0.26 U/mL), BTSR2 (1.635 ± 0.17 U/mL), BTSR3 (0.427 ± 0.06 U/mL), BTSR4 (1.182 ± 0.15 U/mL), BTSR5 (0.558 ± 0.03 U/mL) and BTSR6 (1.273 ± 0.43 U/mL) after 24 hr incubation at 37°C under static condition (Table 2). Among this, highest cellulase activity was expressed by the bacterial isolate identified as Bacillus subtilis BTSR1. So, the strain BTSR1 was considered for the further study. Ojumu et al., [14] investigated about some lignocellulosics, which served as carbon source for the production of cellulase and reported that CMC had a positive influence on the cellulase production. Higher production of cellulase, when CMC served as a substrate may be as a result of induction of the enzyme, since cellulose is known to be a universal inducer of cellulase synthesis [15]. CMC that is the best substrate for cellulase production because it can induce bacteria to produce cellulase enzymes [16]. Screening of potential bacteria from keratinase enzyme activity in basal medium of feather meal broth was screened Brevibacillus parabrevis (1.05 ± 0.35 IU/ml) and Brevibacillus brevis (1.03 ± 0.14IU/ml) at 440 nm in spectrophotometer. Bacillus species were the predominant isolates encountered in this study [17]. Similar results were reported that the cellulosomal cellulases from Bacillus megaterium has both CMCase and avicelase activity; however, the ‘cellulases’ had a higher activity. The mechanisms by which different types of cellulases enhance each other's activities are complex and not completely understood, and the published data are often inconsistent [18]. A moderately thermophilic Bacillus K-12 produced a large amount of cellulase components containing avicelase, xylanase, CMCase and FPase, when grown in avicel medium at 50°C [19].
| S. No | Bacterial isolates | Enzyme activity (U/Ml) |
|---|---|---|
| 1 | BTSR1 | 1.817 ± 0.26 |
| 2 | BTSR2 | 1.635 ± 0.17 |
| 3 | BTSR3 | 0.427 ± 0.06 |
| 4 | BTSR4 | 1.182 ± 0.15 |
| 5 | BTSR5 | 0.558 ± 0.03 |
| 6 | BTSR6 | 1.273 ± 0.43 |
Table 2: Cellulase activity from different isolates.
Purification of cellulase
Extra cellular enzyme was purified to homogeneity from the culture extracts. The supernatant containing extra cellular proteins were separated and extracted by precipitation and it purified by the 40% and 70% ethanol. The cellulolytic protein was 3.27-fold purified with a specific activity of 302.27 U/mg. the total protein content 0.32 mg/mL was obtained from 70% ethanol fraction. 40% ethanol fraction showed the lesser enzyme activity also protein content. In this study, the extracellular cellulose from B. subtilis BTSR1 strain was produced, purified and estimated to be 46 kDa with specific activity was 302.27 U/mg (Table 3). In the previous report, Halophilic A. gracilis isolated from a hypersaline manmade saltern had the potential to produce extracellular amylase, which was extracted, purified and verified by SDS-PAGE analysis, showing a single band of approximately 35 kDa with a specific activity of 131.02 U/mg [20]. An extracellular a-amylase from the obligate halophilic A. penicillioides TISTR3639 strain was produced, purified, and estimated to be 42 kDa by SDS-PAGE with a specific activity of 118.42 U/mg [21].
| Purification step | Total activity (U/mL) | Total protein (mg/mL) | Specific activity (U/mg) | Purification (Fold) |
|---|---|---|---|---|
| Ethanol Fraction 40% | 38.27 | 0.08 | 87.32 | 1.54 |
| Ethanol Fraction 70% | 72.49 | 0.32 | 302.27 | 3.27 |
Table 3: Purification of cellulase from BTSR1 by ethanol fractional precipitation.
Immobilization of enzyme
Immobilization process was standardized with respect to concentration of CaCl2, sodium alginate and bead size in order to obtain maximum efficiency (Figure 2). Since the cross linking between sodium alginate and calcium ions leads to gelation, the gel entrapment of an enzyme depends on the concentration of both, and the effect of concentration of these constituents was investigated on the efficiency of immobilization. Different percentages of sodium alginate gel were tried. However, maximum immobilization efficiency was achieved with 4% alginate with 0.2M CaCl2 and bead size was 10 g. Immobilization of enzymes on suitable supports has been observed to overcome these limitations. The enhanced operational stability of the immobilized enzymes/biocatalysts [22] has been of great significance for their exploitation at commercial level. Besides enhanced stability, on immobilization, enzymes can also acquire additional advantageous properties such as their repeated use and their separation from soluble reaction products and untreated substrate. It also improves their application in solvents. Immobilization often stabilizes the structure of enzymes also, thereby allowing their applications even under harsh environmental conditions of temperature, pH and organic solvents [23]. In previous study Xylanase was entrapped in calcium alginate beads by adding enzyme preparation in sodium alginate drop wise with needles into solution of CaCl2 [24].
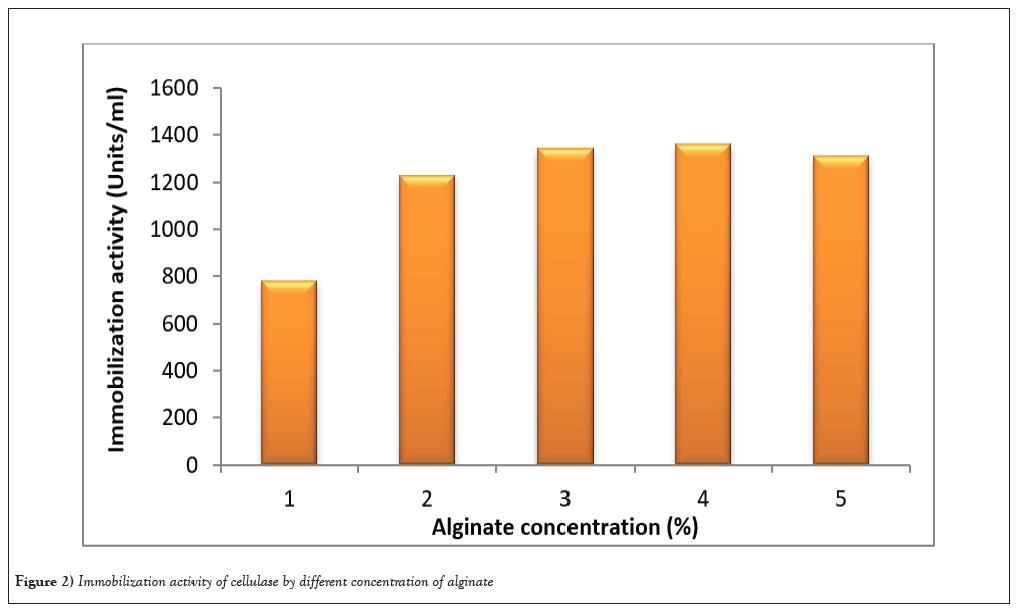
Figure 2: Immobilization activity of cellulase by different concentration of alginate.
Bioethanol production by fermentation
The S. cerevisiae was proved to be very effective in concerting glucose to ethanol in SSF. In this study, bioethanol production was optimized for the highest yield of bioethanol. The substrate wheat bran and coffee pulp were optimized by different concentration from 1-3% (w/v). Immobilized enzymes produced the highest yield of bioethanol 28 g/l was obtained for 4% of substrate concentration and followed by 3% substrate. Free enzymes showed the 17 g/l for 4% substrate (Figure 3). Immobilized cellulase activity nearly doubled the amount provided from the free enzyme activity. Additionally yeast extract concentration also optimized and ethanol production was found to increase 27 g/l of 0.3% yeast extract concentration (Figure 4). pH optimized also play a remarkable role in the production of bioethanol (Figure 5). The highest ethanol production was found to be at pH 5 (21 g/l) and followed by pH 6 (18 g/l). Duration of fermentation was importance for bioethanol production. In this study, highest amount of ethanol yield on 4th day of fermentation (Figure 6). In this study, maximum amount of bioethanol production was observed in the immobilized enzyme than free enzyme. Thus, immobilized condition improved the activity of enzyme to produced bioethanol.

Figure 3: Optimization of bioethanol production by various substrate concentrations of free and immobilized enzymes,  .
.

Figure 4: Optimization of bioethanol production by various yeast extract concentrations of free and immobilized enzymes,  .
.
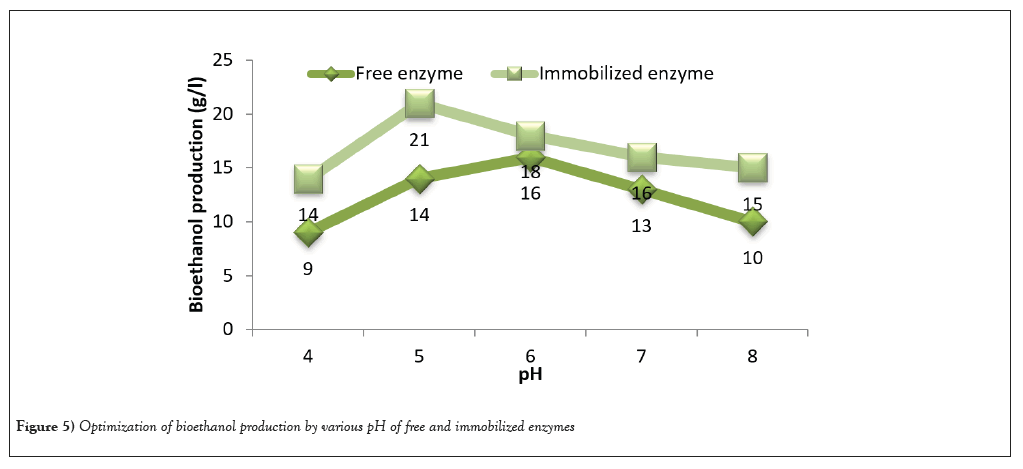
Figure 5: Optimization of bioethanol production by various pH of free and immobilized enzymes.
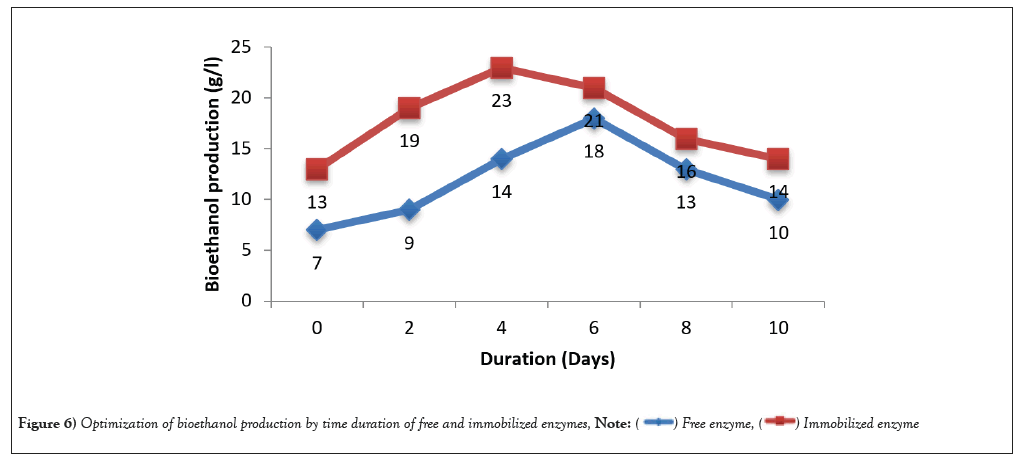
Figure 6: Optimization of bioethanol production by time duration of free and immobilized enzymes,  .
.
In previous study reported that bioethanol production by Aspergillus species by incubating amylase producing fungus and baker’s yeast (S. cerevisiae) at the same time. The evaluated quantity of produced bioethanol was 10.9 g/l within 4 days incubation period [25]. Moreover, F. oxysporum was used for converting starch-rich disposal foods to bioethanol with the aid of S. cerevisiae cultivation, such combinations were developed by two pathways giving a bioethanol production of 16.3 g/l, and simultaneous fermentation (20.6 g/l) of bioethanol production all in one step [26]. In similar study revealed that the highest enzyme yield (165.24 U/mL) was provided by the immobilized amylase with 1% of covalent bonding agents solution, providing double the sugars’ byproduct than from the free amylase form, and double the bioethanol fermentation yield with 0.12 g/g sugar/l. Highly specific activity of such enzyme in the immobilized form could offer a highly effective approach for bioethanol production on the industrial scale than the free and other reported forms [27].
Cellulase enzymes that are formed will break down the cellulose contained in the carbohydrate substrate into glucose. The glucose formed will be used by microbes for growth. These compounds together with ammonium will form protein, therefore, it is necessary to see the relationship between proteins formed by microbes and the activity of enzymes resulting from hydrolysis [28]. According to Itelima et al., [29] that the protein content of fermentation is varied (depending on the raw material used). Reactor level aerobic batch fermentation with optimized process conditions offered a maximum ethanol concentration of 0.06 g/L from 1 g/L of pretreated sugarcane bagasse in [30].
Cellulase is an industrial enzyme which helps in the conversion of lignocellulosic materials into simpler units. In this study, highest cellulase activity was expressed by the bacterial isolate identified as Bacillus subtilis BTSR1. Further, cellulsae enzyme was purified and immobilized with sodium alginate. In addition, the results concluded the higher potency of immobilized cellulase than the free enzyme. Thus, the cellulase immobilized can be effectively used for production of bioethanol.
The authors wish to thanks the Chairman, Maruthupandiyar College, Thanjavur for providing necessary facilities that successful completion of the work.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Sunethra VM, Rajakumar R. Production of bioethanol from free and immobilized cellulase produced by Bacillus subtilis BTSR1. AGBIR.2023;39(6):698-703
Received: 10-Oct-2023, Manuscript No. AGBIR-23-116307; , Pre QC No. AGBIR-23-116307 (PQ); Editor assigned: 13-Oct-2023, Pre QC No. AGBIR-23-116307 (PQ); Reviewed: 27-Oct-2023, QC No. AGBIR-23-116307; Revised: 03-Nov-2023, Manuscript No. AGBIR-23-116307 (R); Published: 10-Nov-2023, DOI: 10.35248/0970-1907.23.39.698-703
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
