Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2024) Volume 40, Issue 2
Through musical and color synaesthesia, the taster builds an associative lineage when encountering a sparkling wine. The so-called “crisp” sound on the surface of a sparkling wine suggests a chromatic anchoring of the color palette. A study of the effect of expedition liqueur on the color change of rose sparkling wine showed that dosage has a direct effect on the color gradient. Dosage technology involves the addition of sulphite components, which protect the sparkling wine from oxidation. Sulphur dioxide, like tannins are natural antiseptics. White and rose wines have a high sulphite content because they are very delicate. The chromatic values of the samples in which the sulphite expedition liqueur was added had color variations from soft pink to copper. The chromatic values of the samples to which the non-sulphite expedition liqueur was added had a clear gradient of shades from frightened nymph thigh color to acajou color.
Expedition liquor; Sparkling wine; Level of sulphites; Chromatic values; Rose wines
The quality of rose wines is primarily determined by their color tonality. The pink color is traditionally considered neutral and represents serenity and equilibrium, transmitting calm and tranquillity and representing a transition between female and male styles. This color varies widely from very pale (sandy or pearly) to bright (pomegranate) shades, right through to orange (apricot) or red (raspberry) depending on the grape variety, terroir and winemaking processes [1]. Chromaticity forms not only the chromatic indices, but also the “musical color”, which is defined not by individual sounds, but by the complete sequence of sounds, the scale, which constitutes a harmony: major or minor.
The musician who established this “sound-and-color” connection was the composer A. N. Scriabin. Having a color-tonal ear, he perceived tonalities in a certain color: C major-red; G major-orange-pink; D major-yellow; A major-green; E major and B major-blue-white colors; F major-bright blue; C major-purple; A major-purple-purple; E major; B major-steel with a metallic sheen and F major-dark red. On the basis of a similar color perception, scriabin combined notation and color scores in his musical compositions.
The tasting ritual starts with color observation, it is the first contact of a taster with wine, the first impression, the first sensation. In the case of rose wines, their color palette is very varied, the color gradient, depending on geographical origin, can be set from north to south. Over the past decade, natural rose sparkling wines have become more transparent and sometimes even stand out as a guarantor of quality. Under pressure from distributors and consumers, many producers have corrected the color of their rose sparkling wines so that they match the color of Provence wines. The color of rose wine is due to the presence of anthocyanin’s, which are very reactive and can undergo reductive-oxidative reactions throughout the fermentation process, affecting the final color of the wine [2]. Some anthocyanin’s have an ortho-diphenol group linked to their aromatic ring (cyanidin, delphinidin and petunidin) which can chelate certain metal cations such as Al3+, Fe3+, Cu2+ and Mg2+ causing a batochrome effect. Anthocyanins are defined as red flavilium cations, but this form predominates only at very low pH values, so they are present mainly in their colorless hydrated hemicetal form. With their maximum absorption at 440 and 460 nm respectively, these pigments directly contribute to the darkening of the pink sparkling wine during the control aging process.
These oxidation reactions lead to the formation of H2O2, which in combination with divalent iron ions (Fe2+) can lead to the formation of reactive oxygen species (such as hydroxyl radicals and hydroxide ions) by means of a reaction commonly known as the fenton reaction [3]. The hydroxyl radical is considered to be one of the most potent non-selective oxidizing agents with the ability to oxidize almost any substance in wine. The oxidation products, mostly ketones and aldehydes are nucleophiles and are considered to be important compounds in the development of color [4]. Compounds such as sulphur dioxide, ascorbic acid and glutathione, because of their action as reducing agents and/or quinone absorbers, are considered important in increasing the oxidation resistance of wine [5].
There are limited information on the composition of rose wines. A study by Wirth et al., [6] aimed at the quantification of polyphenols showed that their proportions are very different from those found in red wines made from the same grape variety. In rose wines, the main phenolic compounds were hydroxycinnamic acids, while anthocyanin’s and flavanols were present in lower concentrations. Higher color intensity and a pinker tone were associated with higher concentrations of anthocyanin’s and phenolic acids and lower pH values [7]. Specific pigment profiles with a lower proportion of coumaroylated formations were observed, reflecting the low extraction rate of these hydrophobic pigments compared to other anthocyanin’s and the higher proportions of pigments obtained, including several groups of pyranoanthocyanins. This study also provided the first report of anthocyanin-caphtharic acid adducts in wines.
The darkening of rose sparkling wines mainly follows a zero-order kinetics [8-10]. Since darkening is mainly related to the oxidation of polyphenols, oxidizable substrates such as o-diphenols and hydroxycinnamic acids are involved to some extent in the oxidation mechanism and many studies have shown that quinone formation has a significant contribution [11].
Expedition liqueur and sparkling wine are prepared from the same wine material, but because the dosage technique involves the addition of sugar and a sulphiting component, the expedition liqueur has a higher concentration of phenolic compounds. During the addition of expedition liqueur, a “browning” of the sparkling wine happens as a result of the maillard reaction [6]. Expeditionary liqueur is traditionally sulphitated to prevent oxidation and, consequently, the finished pink sparkling wine has an orange hue.
The maillard reaction is an enzymatic browning reaction. The reaction happens between the amino groups of amino acids, proteins and the carbonyl groups of reducing sugars, aldehydes or ketones. The effect of buffer solutions on the maillard reaction obtained from reducing sugars accelerates the process considerably with the formation of colour as the reaction starts with the degradation of Amadori compounds (N-substituted 1-amino-1-deoxyketoses-Amadori Reaction Products (ARP)) which are important intermediates in the early stages of the maillard reaction [12,13].
Amadori compounds are formed in the opening phase of the maillard reaction. As a basic rule, the Amadori rearrangement product is always formed by degradation of the schiff base and the latter is obtained by Amadori rearrangement of the corresponding N-glycosylamines [14]. For example, the degradation pathway of ARP, in which various cyclic substances are formed by various mechanisms such as the strecker reaction or ARP acting as nucleophiles and reacting with other sugar molecules to form diketosyl derivatives [15]. The Amadori compound Glucose-Proline (DFP) from reducing sugars, formic acid and 5-Hydroxymethylfurole (HMF) by various routes is the first step in the glucose-proline maillard reaction.
The maillard reaction proceeds in three stages: a starting stage, an intermediate stage and a final stage. The starting stage involves the formation of a sugar amine and Amadori rearrangement, a process that produces colorless products. In the intermediate stage, the colour of the reaction products is weak or yellow, with strong absorption in the ultraviolet region. In the final step, aldol condensation and polymerization of aldehydamides results in the formation of strongly colored compounds [16].
The main reducing sugars involved in the maillard reaction are disaccharides, pentoses and hexoses. The concentration and type of sugar affect the type of products of this reaction. Serine, lysine, arginine and proline react with fructose or glucose to form volatile compounds [17].
In sparkling wine, the result of the maillard reaction is the formation of a number of substances, the so-called markers which give reason to believe that the change of chromatic indices is a result of the process of this reaction [18]. An indication of this fact may be the appearance of a copper tone in the pink sparkling wine, which is maintained by the sulphitation of the expedition liqueur.
During fermentation, sulphur dioxide is a natural by-product of yeast, typically producing less than 30 mg/dm3. Two salt forms of sulphite are commonly used in the wine industry: potassium metabisulphite (K2S2O5) and sodium metabisulphite (Na2S2O5). Potassium metabisulphite dissociates in water into potassium ions (K+) and singly ionised bisulphite (HSO3-). Sulphur dioxide is a bifunctional acid and breaks down into three fractions: molecular sulphur dioxide (SO2); sulphite (SO32-); and bisulphite (HSO3-). The amount of each of these fractions depends on thermodynamic constants and pH. The dissociation process is almost immediate [19].
Bisulphite is the predominant form of free sulphur dioxide at an average pH of 2.8-3.8. It causes inactivation of Polyphenol Oxidase (PPO) enzymes and binding and/or reduction of brown quinones [20]. It is also an effective anthocyanin extractant, but it bleaches the colour and inhibits the polymerization reactions of anthocyanin with other phenols.
Molecular sulphur dioxide exists either as a gas or as single molecules in wine. It is responsible for its antimicrobial and antioxidant activity, it is volatile and is responsible for its smell and sulphurous taste [21].
Flavilium, whose cations react with sulfite to form colorless bisulfite adducts, is involved in the reaction of sulfite bleaching reaction [22]. This reaction is reversible, so that the red flavyl cation can be released from the sulphite adducts. In particular, the hydration equilibrium can be displaced towards the red forms in the presence of associated pigments such as flavonols and hydroxycinnamic acids. This phenomenon is responsible for 30-50% of the colour in young red wines, but may be limited in rosé wines by low pigment concentration. Other anthocyanin reactions described in red wines include the formation of red adducts and colorless adducts which condense with acetaldehyde to give purple adducts bound to methylmethine (ethyl-linked) and react with hydroxycinnamic acids, vinylphenols or carbonyl compounds such as acetaldehyde and pyruvic acid to give different groups of orange pyranoanthocyanins [23]. At an average pH value of 2.8-3.8 for wine, the amount of the sulphite form of sulphur dioxide is minuscule and its reaction with oxygen is very slow.
Part of the sulphur dioxide added to wine will bind with the compounds in the wine the ‘bound’ form and the remainder is called the ‘free’ form. Total sulphur dioxide is the sum of free and bound. The unstable products bound to sulphur dioxide can provide a reserve that fuels the free form when it is deposited by oxidation or evaporation. However, the extent of this redistribution depends on the binding kinetics of the individual products. Binding compounds include carbonyl compounds, ketonic acids, sugars, quinones and anthocyanin’s [24].
The use of sulphur dioxide during fermentation leads to preservation of colour by dissolving anthocyanin’s and polyphenols (the compound anthocyanidin-SO2 is more soluble in aqueous ethanol than anthocyanidin alone). It has also been found that wines fermented with sulphur dioxide retain their colour better [25]. However, too much sulphur dioxide causes a discoloration of the colour gradient, even though this discoloration of the red pigments is reversible. The reduction in must colour is partly explained by the adsorption of anthocyanin molecules onto the walls of the yeast. Most of the colour disappears (60% loss of colour intensity) during the first days of secondary fermentation in the bottle. During the maturation period, the free sulphur dioxide disappears and the anthocyanin’s regain their colour, so that the process is reversible [26].
Anthocyanin pigments bind easily to bisulphite (HSO3-). The colored anthocyanin cation binds to the bisulphite anion to form colorless ancyanin-4-bisulphite [26].
Proanthocyanidins are soluble in ethyl alcohol, insoluble in water and slightly soluble in aqueous-alcoholic solutions such as wine. Therefore, it can be assumed that co-pigmentation in white wines may be a possible mechanism for stabilizing the anthocyanin’s formed if glycosylated flavonols are present as cofactors. However, some authors suggest that co-pigmentation is less significant in rosé wines and probably insignificant in white wines because of low or very low concentrations of anthocyanin’s.
The minimum requirements for the occurrence of co-pigmentation are not fully understood and the formation of these complexes may involve other factors under certain circumstances. At very low concentrations of anthocyanin (about 35 μM) a high ratio of co-pigments to anthocyanin did not lead to a co-pigmentation effect unless metal ions were added [27,28].
As the temperature increases, the amount of free form of sulphur dioxide increases and the bound form decreases. This happens because higher temperatures cause partial dissociation of the bound form which leads to increase of free form and consequently to increase of molecular concentrations of sulphur dioxide [29].
As research materials they used sparkling wine aged cuvee (brut) of Pinot Franc, growing in Noviy Svet (Republic of Crimea, Sudak) and working versions of forwarding liqueur with the following specifications: mass concentration of sugars-70 g/100 cm³, volume fraction of ethanol-11.5%, mass concentration of titratable acids-7.0 g/dm³.
The liqueur production technology involved mixing the high-quality wine material with sucrose by dissolving the latter during mixing and subsequent aging. Dosage of the expedition liqueur based on sugar content: for extra brut-4 g/dm³; for brut (brut)-12 g/dm³; for dry (extra dru)-20 g/dm³; for semi-dry (sec)-35 g/dm³; for semi-sweet (demi sec)-50 g/dm³.
Researches were carried out in laboratory conditions of department of technology of winemaking, fermentative productions, saccharine and food flavoring products of professor A. A. Merzhanian FGBOU VO “KubGTU” on spectrophotometer Unico 1201.
The Unico 1201 spectrophotometer is designed to measure transmission coefficients, optical density and concentration of solutions and is specially designed for wide use in laboratories of all industries.
The principle of operation of the spectrophotometer is based on the comparison of the light flux F0 that has passed through the solvent or comparison solution (blank solution) against which the measurement is made and the light flux F that has passed through the test solution. Light fluxes F0 and F are converted by photodetector into electric signals Uo, U. Also Ut-signal from non-illuminated receiver is measured. According to the values of these signals the microprocessor of the spectrophotometer calculates and displays the measurement result in the units of transmission coefficient, optical density or concentration, depending on the selected measurement mode.
Calculation of spectrophotometric indicators
Many scientific studies have been devoted to the analysis of the colour characteristics of wine. The colour of white wines can be estimated based on the contribution of the two components red and yellow pigment and of red wines based on the proportion of the three pigments red, yellow and blue.
The optical characteristics are expressed in terms of Intensity(I), hue(T) and yellowness(G).
The method is based on a spectrophotometric method which allows the calculation of tristimulus values and trichromatic coefficients required for colour designation (International Standardization Series (ISS), Commission Internationale de l'Eclairage (CIE)). The proportion of yellow pigment D420 (optical density of the wine layer thickness at 420 nm) depends on the mass concentration of degradation products of tannins and anthocyanin’s. The contribution of the red component (D520) is provided by the content of free anthocyanin’s in the form of flavilium cations and the anthocyanin-tannin complex. The blue pigment (D620) is in turn formed by free anthocyanin’s in quinone form or by a tannin and anthocyanin complex.
The relation of the spectrophotometric indices to the organoleptic and their classification for the evaluation of quality are presented in the P/sudraud method in 1958 [30]. He proposed two indices for calculation: hue and intensity, expressed at wavelengths of 420 and 520 nm according to formula 1 and 2:
I = D420 + D520 ……………. (1)
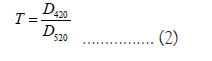
Initially, the two-component colour of rose sparkling wines was assumed to be produced by monomeric anthocyanin’s and colored condensation products of phenolic substances, which are characterised by absorption maxima at 520 and 420 nm wavelengths respectively.
The Y. Glories takes into account the role of blue pigments in the colour complexity of rosé sparkling wines, where the colour intensity is expressed by formula 3:
I = D420 + D520+D620 ……………. (3)
A correlation has been established between the value of colour parameters and the content of phenolic compounds. By methods of regression analysis equations were obtained, allowing to calculate colour coordinates L, a and b on the basis of Y. Glories and optical density values of 420, 520, 620 nm. On the basis of coordinates X, Y, Z and formula (4) it is possible to calculate the value of yellowness index (G) which characterizes the intensity of yellow and brown colour shades of wine materials and wines:

where X, Y, Z are coordinates in CIE system.
In turn, the X, Y and Z coordinates are calculated using formulae 5-7:
X = 0, 42T625 + 0,35T550 + 0, 21T445 ……………. (5)
Y = 0, 20T625 + 0,63T550 + 0,17T495 ……………. (6)
Z = 0, 24T495 + 0,94T445 ……………. (7)
Expedition liqueur is a sugar-containing product. Prepared from bottled wine after secondary fermentation with the addition of highly refined white sugar (without betaine alkaloid), citric acid and the use of sulphiting components. Applying the semantic concept, rose wine can be described by the intensity or tone of pink (pale, grey, purple, orange, etc.) or with terminology denoting objects of the same colour (apricot, salmon, raspberry, etc.). Spectrophotomeric analysis has the prestige of offering an objective measure of colour perceived by the human eye.
Instrumental method
According to the experimental results received by us at doses of expeditionary liqueur for reception of sparkling wine extra brut, brut, dry, semi-dry and semi-sweet in experimental samples, it is established that the value of I increases in proportion to addition of expeditionary liqueur: sample no.1 has value 0,266 units and sample no. 6 has value 0,369.
It is established, that the colour intensity I for pink sparkling wine is less than 0.5, for slightly colored red sparkling wine-0.5-1.0, for well coloured-1.0-2.0, for intensively colored more than 2.0. All samples have a colour index of less than 0.5.
The colour tone index T indicates the intensity in the coloring of yellow-brown tones, formed under the influence of condensation products of phenolic substances. So in sample no.1 the value is 1.69 and in sample no.6 the value is 1.44. At value of the index less than 0, 8 the colour of wine is characterized as violet, in a range of values 0.8-1.2-red, at T>1.2-orange.
The value of G is in a range from 21.4 to 24.9, we observe increase of value from sample no.1 to sample no.6, accordingly the forwarding liquor significantly influences values of colour parameters (Figures 1 and 2).
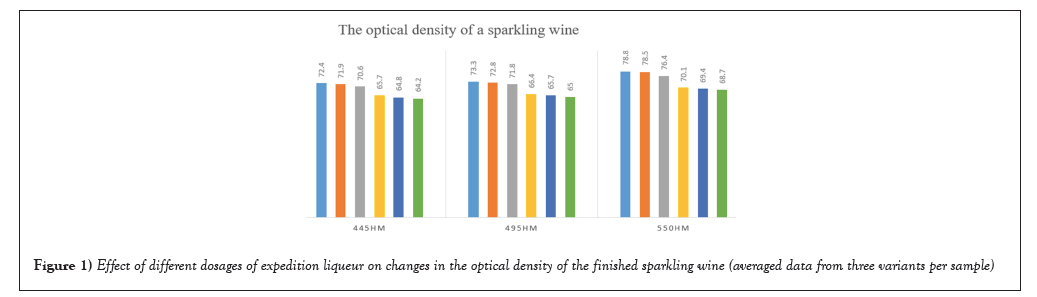
Figure 1: Effect of different dosages of expedition liqueur on changes in the optical density of the finished sparkling wine (averaged data from three variants per sample).
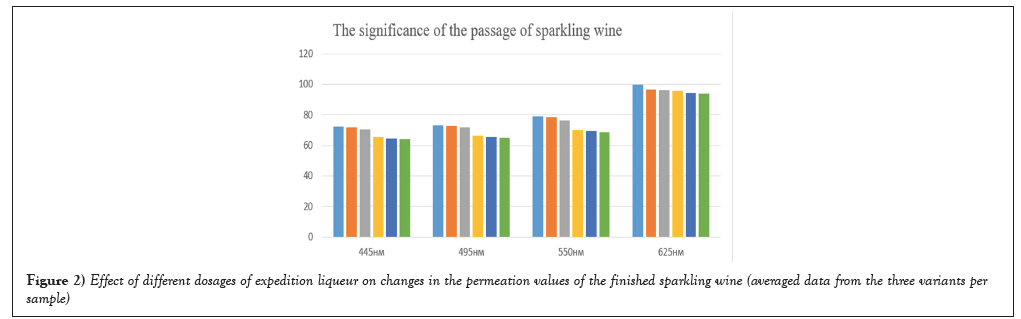
Figure 2: Effect of different dosages of expedition liqueur on changes in the permeation values of the finished sparkling wine (averaged data from the three variants per sample).
Spectrophotometric or colorimetric colour analysis is accurate but not available to all. A paper colour chart representing the main colors of French rose wines has been developed, IV France group national rose. It consists of 143 colour charts which differ in tone (nuance) and saturation (strength of colour sense) [31]. The colour blocks make it possible to quantify colour in terms of intensity.
Glutathione is a tripeptide found naturally in grapes and wine. This substance is directly linked to the oxidation process, where it plays an important role as an antioxidant. The sulfhydryl group in its molecule acts as a nucleophile capable of replacing the electrophilic O-quinone ring and thus regenerating the dihydroxy-ring [32,33].
Visual method
For visual evaluation, two test samples of expedition liqueur from Pinot Franc ("Crimea wine") were prepared, which had the following characteristics: 11.30% vol. of ethyl alcohol; 1.6 g/dm3 mass concentration of sugars and 6.3 g/dm3 mass concentration of titratable acids.
The first liquor sample had a calculated amount of “Solfosol M” sulphiting agent added, the second sample without the addition of sulphiting agent SO2 content: 150 g/dm3 (15% weight/volume).
The rate of spending of “Solfosol M”, which goes to the sulphitization of the expedition liquor before the feeding for degorge, is made according to the calculation of the marks. The quantity of the solution is calculated for the yield volume of ready sparkling wine of a certain brand: extra-bright-0,0004 ml; brut-0,0012 ml; dry-0,0020 ml; semi-dry-0,0035 ml and semi-sweet-0,0050 ml. Six experimental samples by marks were prepared.
In the samples, a change in chromatic indices from soft pink (sample no. 1) to copper (sample no. 6) was observed. Samples no. 1-4 are almost identical because of the binding of pigments with bisulphite (HSO3-) and an increase in pH, which prolongs the formation of colorless carbinol pseudobases and chalcone structures followed by the formation of anionic quinoidic particles. This is related to the kinetic and thermodynamic competition between the hydration reaction of the flavilium ion. At pH 4-5 the anthocyanin solution has a very weak tone because of the small amount of flavillium cation and quinoid anion. Pink wines with an orange tone, represent levels of high hydroxycinnamic acid and low levels of glutathione. Such wines go through intensive oxidation mechanisms and, as a consequence, browning of the must and wine as a result of sulphitization occurs (Figures 3 and 4).

Figure 3: Chromatic values of the Pinot Franc sparkling wine after the addition of sulphite liqueur, from above.

Figure 4: Chromatic characteristics of the Pinot Franc sparkling wine after the addition of sulphite liqueur, full-face view.
Experimental samples have a clear gradient of colour palette: from sample no.1 the colour of the color of the frightened nymph’s thigh (the lightest shade of gently pink) to sample no.6 the colour of acaju (the colour of “redwood”). Without the application of sulphite, the colour tonality changed according to a series of fibonacci levels, as confirmed by the colour descriptor for rose sparkling wine. In this case, at low pH (pH<3) the flavilium cation and the quinoid base appear red, while the quinoid monoanion and dianion can be seen in purple and blue. Peonidine (3-O-methylated anthocyanin) has a cherry red tone at low pH (sample no. 6), while at pH 4-5 it is stable and has a blue hue (sample no. 1) [34]. Acylation of anthocyanin, which happens without the presence of sulphur dioxide, increases the proportion of flavillin cations and helps to maintain the redness of the anthocyanin pigment (Figures 5 and 6).

Figure 5: Chromatic values of the Pinot Franc sparkling wine after the addition of unsulphured liqueur, from above.
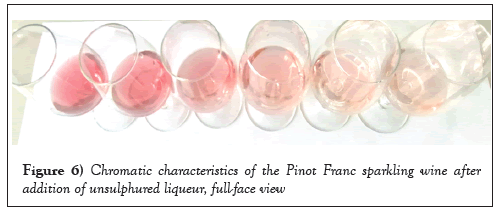
Figure 6: Chromatic characteristics of the Pinot Franc sparkling wine after addition of unsulphured liqueur, full-face view.
Experimental samples in which sulphated liqueur was added had a lower appeal from the consumer’s point of view. If this group of samples were considered in terms of ‘musical coloring’, it could be argued that the sound of this sparkling would be in the ‘G-major’. Chemically, the higher hydroxycinnamic acid content and the lower glutathione content give an intense tint to the D420 yellow pigment at the expense of a higher mass concentration of degradation products of tannins and anthocyanin’s.
From the consumer’s point of view, the experimental samples in which unsulphured liqueur has been added meet the main market trends and satisfy the need for a “beautiful” gently pink sparkling wine in line with the wines of Provence. Considering this group in terms of “musical coloring”, the sound of this sparkling wine will be in “F-sharp major” (brass). From the chemical point of view, the low pH allowed the proportions of yellow D420, red D520 and blue D620 pigments to react in a synergistic way, which allowed mutually reinforcing gradient tones in accordance with chromatic indices.
Activation of glucose-proline reaction of maillard is observed at dosing of expeditionary liqueur at a rate more than 50 g/dm³ and can be caused by the increased concentration level of reduced sugars which by means of shift of equilibrium actively enter into reaction of saccharoamino condensation of carbohydrates and proteins, namely glucose-proline reaction of maillard.
This fact is indirectly confirmed by the results of organoleptic analysis, according to which in the sparkling pink semi-sweet Pinot Franc wine, in which sulphured expedition liqueur was added, the copper shade of colour prevails (colour of the crimean sunset). At the same time, in the samples of sparkling pink extra brut, brut, semi-dry, dry wine and no colour was identified.
It has thus been established that the addition of expedition liqueur generally contributes to a change in the colour tones of the finished sparkling wine and that organoleptic appreciation and visual appeal depend on its addition. The colour of rosé wine consists of a broad chromatic palette, ranging from “pink marble” to “crimson” tones. On the basis of the analysis of the results it can be stated that by regulating the dosage of the expedition liqueur in the production of sparkling wines it is possible to influence the initiation and course of the sugar-amin reaction of maillard, which allows to shape and correct the chromatic qualities of a sparkling wine in a targeted way.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Igorevna TV, Vladimirovna OI, Evgenievna SV. Practical aspects of regulating the chromatic indices of rose sparkling wine by expedition liqueur with the use of sulphiting agents. AGBIR.2024;40(2):968-973.
Received: 16-Feb-2024, Manuscript No. AGBIR-24-127687; , Pre QC No. AGBIR-24-127687 (PQ); Editor assigned: 19-Feb-2024, Pre QC No. AGBIR-24-127687 (PQ); Reviewed: 04-Mar-2024, QC No. AGBIR-24-127687; Revised: 11-Mar-2024, Manuscript No. AGBIR-24-127687 (R); Published: 18-Mar-2024, DOI: 10.35248/0970-1907.24.40.968-973
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
