Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2023) Volume 39, Issue 6
The nodal segment, shoot apex, and leaf explant of the Sindhuri cultivar of Punica granatum were planted on MS Medium supplemented with varying concentrations of auxins and cytokinins single or in combination in order to induce calluses and shoot buds. The best media to induce shoot buds in nodal segment explants was found to be MS media supplemented with 2.0 mg/l BAP. The highest degree of shoot bud induction in shoot apex explants was observed with MS media having 2.5 mg/l BAP. Rich callus induction was accomplished in shoot apex explants by utilizing MS medium supplemented with 2.0 mg/l BAP. The best media for inducing callus in leaf explants was found to be MS media supplemented with 1.0 mg/l BAP+1.0 mg/l NAA.
Callus; In vitro; Nodel segment; Pomegranate; Shoot apex
The family "Punicaceae" includes the pomegranate (Punica granatum L.). It originated in Iran and spread over Asia, Africa, and Europe's Mediterranean region. It contains 2n=2x=16/18 chromosomes. India leads the globe in pomegranate production. India has seen a notable expansion in both area and production during the past ten years. During this time, India's pomegranate exports have increased by 3.5 times [1-4].
North Indian states that have recently begun to cultivate pomegranates are recommended to investigate the viability of doing so in non-traditional locations as this could lead to increased yield. The economic standing of the farmers in these states would greatly benefit from this. Despite being the world's largest producer, India's export share of pomegranates is just about 14% of the total, compared to China's 34% and Iran's 29%, who both have 50% and 33% less land than India [5].
In Rajasthan, pomegranate cultivation is quite popular, and Sindhuri is one of the prominent varieties grown in the region. The primary producing districts of pomegranates are Barmer, Jalore, Jodhpur and Chittorgarh. Micro propagation techniques can be particularly useful in maintaining the genetic purity and health of these plants. The aim of the current research is to evolve a dependable methodology for in vitro shoot and callus induction from nodel segment, shoot apex, and leaf explants in order to generate true-to-type plants.
The Punica granatum cv. Sindhuri was the subject of the current study research. The Department of PBG, Sri Karan Narendra COA, Jobner, provided shoot apexes, nodal segments and leaves for use as explants from healthy trees. Every chemical utilized in this investigation was of analytical quality. During the investigation, Skoog Medium and Murashige were used. Various surface sterilization treatments were used to sterilize each explant. Leaves, shoot apexes, and nodal segments with two to three nodes were gathered and utilized as explants. Following that, the explants were properly cleaned for 20 minutes under running water and then for 10 minutes while being vigorously shaken, they were cleaned with liquid detergent (RanKleen). Explants were first cleaned with detergent and then again for five minutes to get rid of any remaining soap residue under running tap water. Antioxidant solution treatment (150 mg/l ascorbic acid and 100 mg/L polyvinylpyrrolidone) was applied to isolated nodal segments by soaking them in the solution for 20 minutes at a time under a laminar air flow hood, followed by 3 washes with sterile double distilled water. After 45 minutes in a laminar air flow hood with a fungicide (Bavistin) solution (1 mg/l), nodal segments were again washed three times with sterile double distilled water. Lastly, explants were placed in a laminar air flow cabinet and surface sterilized with 0.1 percent HgCl2. While shoot apex and leaves were sterilized for three to four minutes and one to two minutes, respectively, nodal segments were sterilized for five to six minutes. After giving them a complete four to five rounds of washing in sterile double-distilled water, these were injected into culture media that had been enhanced with different amounts of plant growth regulators. Under fluorescent lighting, all cultures were incubated at 25+2°C during a photoperiod of 14:10.
Plant growth regulators
Direct shoot proliferation: To promote direct shoot proliferation from the nodal segment and shoot apex, various concentrations of plant growth regulators were added to the MS Medium both individually and in combinations.
1. Individual PGRs supplemented to the media.
BAP/Kn and NAA/IBA: 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mg/l
2. Plant growth regulators combined or added
(1.0 and 2.0 mg/l) BAP/Kn+(1.0 and 2.0 mg/l) NAA/IAA
3. The control in each experiment was MS basal medium supplemented with no growth regulator.
Induction of callus: In order to induce callus, shoot apex and leave explants were injected into a medium supplemented with varying concentrations of PGR.
1. Individual PGRs supplemented to the media.
BAP/Kn & NAA/IBA: 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mg/l
2. Plant growth regulators combined or added
(1.0 and 2.0 mg/l) BAP/Kn+(1.0 and 2.0 mg/l) NAA/IAA
3. The control in each experiment was MS basal medium supplemented with no growth regulator.
The study employed a CRD and the data were subjected to standard error and mean analysis in accordance with Snedecor and Cochran's [6] methodology. After transforming the values for each replication's shoot and root induction from explants using square root transformation, the standard error for each number of induction was determined as follows:
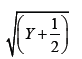
Where, Y=original value.
Impact of PGRs
These explants reacted differently when transferred on media supplemented with varying doses of PGRs.
Impact of cytokinins
Different pomegranate explants responded differently to cytokinins. In leaf and shoot apex explants, callus inductions were primarily seen and in shoot apex and nodal segment explants, shoot bud inductions.
Impact of single-dose BAP added in medium (0.5-3.0 mg/l): Within 16 to 18 days of inoculation, the nodal segments were initiated to stimulate shoot bud proliferation at all BAP levels (0.5-3.0 mg/l). Induction of the maximum number of shoot buds (1.9) was noted at 2.0 mg/l BAP with 100% frequency (Table 1 and Figure 1). Shoot apex explants were injected with varying concentrations of BAP (0.5-3.0 mg/l) on MS media. Within two to three weeks of incubation, callus and shoot buds were seen at all levels. At 2.5 mg/l BAP, the highest number of shoot bud induction (2.3) was seen with 100% frequency (Figure 2). Following 16 to 20 days of incubation, the base of shoot apex explants incubated at varying BAP concentrations (0.5-3.0 mg/l) likewise developed a small light green, semi-compact callus. The fresh callus weight that was highest (0.80 g) was recorded at a BAP level of 2.0 mg/l, while the lowest (0.57 g) was recorded at a BAP level of 0.5 mg/l and 3.0 mg/l. The abundant callus did not exhibit any morphogenesis and differentiated at 2.0 mg/l BAP when it was placed on repeated sub cultures at different levels of BAP. Upon microscopic analysis, only loose parenchymatous cells were visible in this callus.
| Concentration (mg/l) | Response (%) | Callus | Shoot multiplication | ||||
|---|---|---|---|---|---|---|---|
| Days (taken) for callus initiation | Fresh callus weight (g) | Days (taken) for sprouting | Number of shoot buds/explant | Shoot length (cm) | Response (%) | ||
| Nodal segment explant | |||||||
| 0.5 | - | - | - | 17.5 | 1.2 ± 0.15 | 6.08 ± 0.12 | 100 |
| 1 | - | - | - | 17.1 | 1.3 ± 0.15 | 6.28 ± 0.07 | 100 |
| 1.5 | - | - | - | 17.2 | 1.3 ± 0.13 | 6.35 ± 0.12 | 100 |
| 2 | - | - | - | 17.5 | 1.9 ± 0.20 | 6.87 ± 0.11 | 100 |
| 2.5 | - | - | - | 16.3 | 1.4 ± 0.15 | 6.22 ± 0.12 | 100 |
| 3 | - | - | - | 17.4 | 1.3 ± 0.10 | 5.94 ± 0.08 | 100 |
| Shoot apex explant | |||||||
| 0.5 | 100 | 17.7 | 0.57 (+) | 12.4 | 1.7 ± 0.15 | 5.93 ± 0.09 | 100 |
| 1 | 100 | 18.5 | 0.60 (++) | 13.1 | 1.7 ± 0.15 | 6.00 ± 0.13 | 100 |
| 1.5 | 100 | 17.2 | 0.60 (++) | 13.2 | 1.8 ± 0.13 | 6.15 ± 0.08 | 100 |
| 2 | 100 | 19 | 0.80 (+++) | 12.8 | 2.2 ± 0.20 | 6.27 ± 0.15 | 100 |
| 2.5 | 100 | 18 | 0.63 (++) | 13.5 | 2.3 ± 0.15 | 6.42 ± 0.09 | 100 |
| 3 | 100 | 17.1 | 0.57 (+) | 14.5 | 1.9 ± 0.10 | 5.86 ± 0.09 | 100 |
| Leaf explant | |||||||
| 0.5 | 90 | 27.9 | 0.55 (+) | - | - | - | - |
| 1 | 100 | 27.1 | 0.58 (+) | - | - | - | - |
| 1.5 | 100 | 24.9 | 0.77 (+++) | - | - | - | - |
| 2 | 90 | 25.8 | 0.62 (++) | - | - | - | - |
| 2.5 | 80 | 26.3 | 0.59 (+) | - | - | - | - |
| 3 | 70 | 27 | 0.54 (+) | - | - | - | - |
Note: +++=Profuse callus, ++=Medium callus, +=Slight callus.
Table 1: Morphogenetic effects of varying concentrations of BAP (cytokinin) applied separately in the MS medium on nodal segment, shoot apex, and leaf explants among other explants.

Figure 1: Induction of shoot buds in a nodal segment explant on medium (MS) enhanced with BAP @ 2.0 mg/l.

Figure 2: Initiation of shoot buds in the shoot apex explant on medium (MS) enriched with BAP @ 2.5 mg/l.
The reaction of the leaf explants was different from that of the shoot apex and the nodal segment; only callus multiplied. After the injection, callus induction began 24 to 28 days later. 0.5-3.0 mg/l BAP was used to create light green callus. With 100% frequency at 1.5 mg/l BAP, the highest callus weight (0.77 g) was recorded, and 2.0 mg/l BAP (0.62 g) came next (Table 1 and Figure 3).
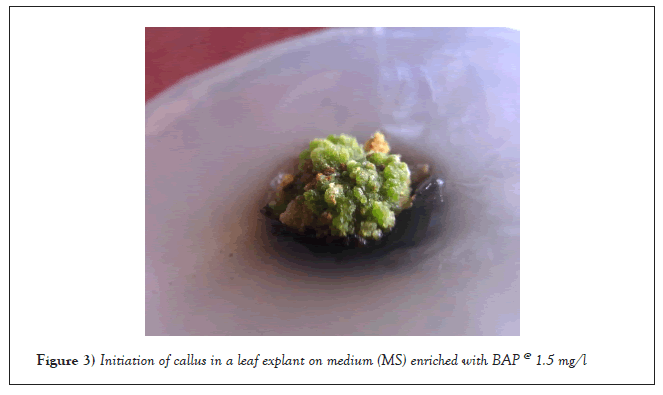
Figure 3: Initiation of callus in a leaf explant on medium (MS) enriched with BAP @ 1.5 mg/l.
Impact of Kn applied separately in medium (0.5-3.0 mg/l): Within 15 to 17 days of inoculation, nodal explants at all Kn doses (0.5-3.0 mg/l) began to grow. At 2.5 mg/l, the maximum shoot bud (1.7) was seen with 100% frequency (Table 2 and Figure 4). After two weeks of incubation, the shoot apex explants began to develop in kinetin enriched media. Similar to BAP, shoot bud induction was seen in 14-17 days at all kinetin doses. With 100% frequency, the maximum shoot bud induction (1.7) was noted at the 2.5 mg/l Kn level (Figure 5). Within 14-17 days of inoculation at all Kn levels, callus induction was initiated in the shoot apex explant in the kinetin supplemented media. The highest callus induction of 0.73 g was noted at a frequency of 100% with 2.0 mg/l Kn. With rising Kn levels, callus induction and its frequency gradually increased until declining at higher levels (Table 2). Within 8 to 12 days of inoculation, an increase in leaf size was observed in leaf explants cultured on MS medium supplemented with varying levels of Kn. At all Kn levels, callus induction was seen on leaf explants. At 1.5 mg/l Kn, the highest brownish green callus proliferation (0.70 g) was seen with 100% frequency (Figure 6).
| Concentration (mg/l) | Response (%) | Callus | Shoot multiplication | ||||
|---|---|---|---|---|---|---|---|
| Days (taken) for callus initiation | Fresh callus weight (g) | Days (taken) for sprouting | Number of shoot buds /explant | Shoot length (cm) | Response (%) | ||
| Nodal segment explant | |||||||
| 0.5 | - | - | - | 17 | 1.3 ± 0.15 | 5.96 ± 0.08 | 100 |
| 1 | - | - | - | 15.3 | 1.4 ± 0.16 | 6.16 ± 0.08 | 100 |
| 1.5 | - | - | - | 16.7 | 1.4 ± 0.16 | 6.26 ± 0.11 | 100 |
| 2 | - | - | - | 16.3 | 1.5 ± 0.17 | 6.15 ± 0.07 | 100 |
| 2.5 | - | - | - | 15.8 | 1.7 ± 0.15 | 6.45 ± 0.08 | 100 |
| 3 | - | - | - | 16.5 | 1.5 ± 0.16 | 5.88 ± 0.09 | 100 |
| Shoot apex explant | |||||||
| 0.5 | 60 | 16.8 | 0.56 (+) | 10.9 | 1.2 ± 0.13 | 5.94 ± 0.24 | 80 |
| 1 | 80 | 16 | 0.58 (+) | 11.3 | 1.3 ± 0.15 | 6.19 ± 0.20 | 90 |
| 1.5 | 100 | 15.7 | 0.60 (++) | 10.5 | 1.3 ± 0.15 | 6.16 ± 0.21 | 90 |
| 2 | 100 | 16 | 0.73 (+++) | 11.4 | 1.5 ± 0.16 | 6.10 ± 0.09 | 100 |
| 2.5 | 100 | 15.1 | 0.63 (++) | 11.8 | 1.7 ± 0.15 | 6.54 ± 0.06 | 100 |
| 3 | 80 | 14.5 | 0.61(++) | 11.9 | 1.3 ± 0.15 | 5.99 ± 0.23 | 90 |
| Leaf explant | |||||||
| 0.5 | 70 | 31.2 | 0.59 (+) | - | - | - | - |
| 1 | 100 | 32.7 | 0.62 (++) | - | - | - | - |
| 1.5 | 100 | 31 | 0.70 (+++) | - | - | - | - |
| 2 | 100 | 33.1 | 0.61 (++) | - | - | - | - |
| 2.5 | 100 | 30.7 | 0.61 (++) | - | - | - | - |
| 3 | 100 | 29.5 | 0.55 (+) | - | - | - | - |
Note: +++=Profuse callus, ++=Medium callus, +=Slight callus.
Table 2: The morphogenetic impact of different cytokinin (Kn) concentrations added separately in the medium (MS) on distinct explants, such as the nodal segment, shoot apex, and leaf.

Figure 4: Initiation of shoot buds in a nodal segment explant on media (MS) enriched with Kn @ 2.5 mg/l.
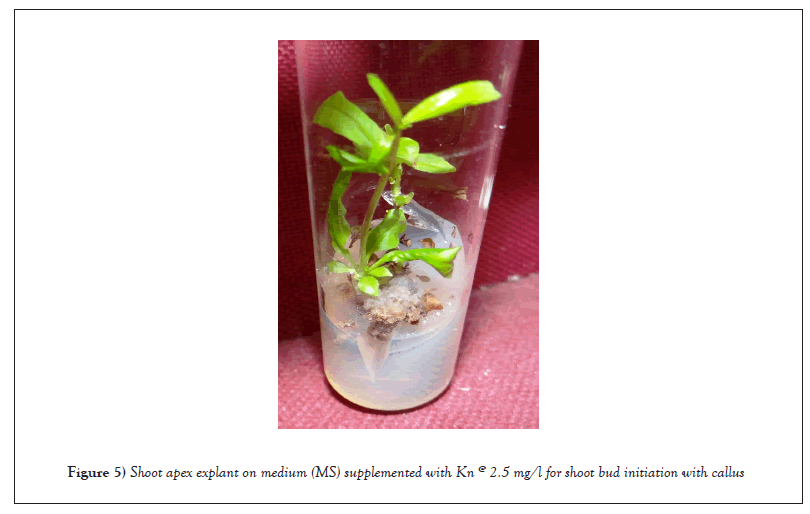
Figure 5: Shoot apex explant on medium (MS) supplemented with Kn @ 2.5 mg/l for shoot bud initiation with callus.

Figure 6: Initiation of callus in leaf explants on media (MS) enriched with Kn @ 1.5 mg/l.
Effect of auxins
Auxins produced varying morphogenic responses in the nodal segment, shoot apex, and leaf explants. For every explant of the pomegranate cultivar Sindhuri, there was a predominant observation of shoot multiplication/callus and/or root induction.
Impact of a single, medium-added dose (0.5-3.0 mg/l) of IBA: Upon incubating nodal segments on MS medium added with varying IBA concentrations, shoot bud induction was initially detected at 13 to 16 days’ post-inoculation at all IBA concentrations (0.5-3.0 mg/l). At 1.5 mg/l IBA, the highest number of shoot buds (1.7) were seen with 100% frequency. Conversely, between 2.5 and 3.0 mg/l IBA, the fewest shoot buds (1.3) were noted. By increasing IBA concentration to 1.5 mg/l improved the induction of shoot buds. Nevertheless, the induction of shoot buds was gradually inhibited as the concentration of IBA increased (>1.5 mg/l). The length of the shoots was likewise impacted by varying IBA concentrations. The dose of 1.5 mg/l IBA produced the longest shoot (6.32 cm), which was followed by 2.5 mg/l IBA (6.17 cm). Lowest shoot length (5.81cm) was observed at 3.0 mg/l (Table 3). Shoot apex explants were injected with varying concentrations of IBA (0.5-3.0 mg/l) on MS media. Root induction, callus, and shoot buds were mostly seen. Within 13-17 days of the inoculation, callus induction was initiated at the base of the shoot apex explant. At 100% frequency, 1.0 mg/l IBA produced the highest callus induction (0.66 g), which was followed by 1.5 mg/l (0.61 g). At 1.0 mg/l IBA, the highest shoot bud (1.6) induction was seen, and at 100% frequency, 1.5 mg/l IBA was seen 1.5 mg/l IBA produced the maximum shoot length (6.0 cm), which was followed by 2.5 mg/l IBA (5.76 cm). Similar responses to shoot length were seen when the level of IBA was increased, as was the case with shoot bud induction at various IBA levels. At 1.0 mg/l IBA, there was abundant root induction; however, in shoot apex explants, root induction was totally suppressed at higher IBA concentrations (Table 3).
| Concentration (mg/l) | Response (%) | Callus | Shoot multiplication | ||||
|---|---|---|---|---|---|---|---|
| Days (taken) for callus initiation | Callus (Fresh) weight (g) | Days (taken) for sprouting | Number of shoot buds/explant | Shoot length (cm) | Response (%) | ||
| Nodal segment explant | |||||||
| 0.5 | - | - | - | 15.2 | 1.4 ± 0.16 | 5.93 ± 0.10 | 70 |
| 1 | 40 | 21.5 | (R++)6.5 ± 0.03 | 15.1 | 1.4 ± 0.16 | 6.03 ± 0.08 | 80 |
| 1.5 | - | - | - | 14.5 | 1.7 ± 0.15 | 6.32 ± 0.04 | 100 |
| 2 | - | - | - | 13.6 | 1.6 ± 0.16 | 6.02 ± 0.09 | 100 |
| 2.5 | - | - | - | 14.8 | 1.3 ± 0.15 | 6.17 ± 0.06 | 70 |
| 3 | - | - | - | 15.2 | 1.3 ± 0.15 | 5.81 ± 0.010 | 60 |
| Shoot apex explant | |||||||
| 0.5 | 100 | 16.8 | 0.57 (+) | 11.5 | 1.4 ± 0.16 R+ | 5.24 ± 0.10 | 100 |
| 1 | 100 | 15.9 | 0.66 (++) | 11.3 | 1.6 ± 0.16 R+++ | 5.74 ± 0.09 | 100 |
| 1.5 | 100 | 15.6 | 0.61 (++) | 10.9 | 1.5 ± 0.17 R++ | 6.00 ± 0.08 | 100 |
| 2 | 80 | 15.2 | 0.60 (++) | 10.2 | 1.4 ± 0.16 R+ | 5.75 ± 0.05 | 100 |
| 2.5 | 70 | 13.9 | 0.59 (+) | 11.8 | 1.3 ± 0.15 -- | 5.76 ± 0.10 | 100 |
| 3 | 60 | 14 | 0.57 (+) | 10.9 | 1.3 ± 0.15 -- | 5.49 ± 0.06 | 100 |
| Leaf explant | |||||||
| 0.5 | 100 | 26 | 0.60 (++) | - | - | - | - |
| 1 | 100 | 26.8 | 0.62 (++) | - | - | - | - |
| 1.5 | 100 | 27.7 | 0.64 (++) | - | - | - | - |
| 2 | 100 | 27.3 | 0.75 (+++) | - | - | - | - |
| 2.5 | 100 | 27.2 | 0.64 (++) | - | - | - | - |
| 3 | 100 | 26.1 | 0.62 (++) | - | - | - | - |
Note: +=Slight callus, ++=Medium callus, +++=Profuse callus, R+= Slight roots, R++= Medium roots, R+++=Profuse roots.
Table 3: The morphogenetic impact of varied auxin (IBA) concentrations added separately in the medium (MS) on distinct explants, such as the nodal segments, shoot apexes, and leaves.
Within 26 to 28 days of inoculation, leaf explants on IBA-supplemented medium showed the induction of light brown greenish callus at all IBA concentrations (0.5-3.0 mg/l). At 2.0 mg/l IBA, 100% frequency allowed for the observation of the maximum callus proliferation (0.75 g). Callus growth was reduced with further increases in IBA in leaf explants (Table 3).
Impact of single-dose NAA (0.5–3.0 mg/l) given to medium: Within 14 to 16 days after inoculation, shoot bud induction was initiated in the nodal segment at all NAA concentrations (0.5-3.0 mg/l). A maximum of 1.6 shoot buds was detected at a frequency of 100% when using 2.0 mg/l NAA (Table 4). When the shoot apex explant was injected into MS media that had varying NAA concentrations added to it (0.5-3.0 mg/l). At every NAA level, callus, shoot buds, and roots were all observed with 100% frequency, with the exception of the root. Within 14 to 16 days of inoculation, light green callus induction was initiated at the base of the shoot apex explant. At 2.0 mg/NAA, the highest callus weight (0.67 g) was noted. At 1.5 mg/l NAA, the longest shoot length (6.34 cm) was recorded, and it was followed by 0.5 mg/l (6.06 cm). The length of the shoot buds was shortened by raising the NAA levels (>1.5 mg/l). 0.5 mg/l NAA showed abundant roots, while greater concentrations entirely suppress it (Table 4).
| Concentration (mg/l) | Response (%) | Callus | Shoot multiplication | ||||
|---|---|---|---|---|---|---|---|
| Days (taken) for callus initiation | Callus (Fresh) weight (g) | Days (taken) for sprouting | Number of shoot buds/explant | Shoot length (cm) | Response (%) | ||
| Nodal segment explant | |||||||
| 0.5 | - | - | - | 15.1 | 1.2 ± 0.13 | 6.18 ± 0.07 | 100 |
| 1 | - | - | - | 14.4 | 1.4 ± 0.16 | 6.16 ± 0.07 | 100 |
| 1.5 | - | - | - | 14.7 | 1.4 ± 0.16 | 6.22 ± 0.10 | 100 |
| 2 | - | - | - | 15 | 1.6 ± 0.17 | 6.44 ± 0.10 | 100 |
| 2.5 | - | - | - | 14.1 | 1.5 ± 0.15 | 6.12 ± 0.06 | 100 |
| 3 | - | - | - | 14.7 | 1.3 ± 0.16 | 5.66 ± 0.10 | 100 |
| Shoot apex explant | |||||||
| 0.5 | 100 | 16.3 | 0.58 (+) | 12.3 | 1.3 ± 0.15 R+++ | 6.06 ± 0.09 | 100 |
| 1 | 100 | 15.1 | 0.62 (++) | 10.2 | 1.3 ± 0.15 R+ | 5.98 ± 0.14 | 100 |
| 1.5 | 100 | 14.3 | 0.63 (++) | 10.6 | 1.4 ± 0.16 R+ | 6.34 ± 0.08 | 100 |
| 2 | 100 | 15.6 | 0.67 (++) | 10.6 | 1.7 ± 0.15 - | 5.87 ± 0.10 | 100 |
| 2.5 | 100 | 13.4 | 0.60 (++) | 11.2 | 1.5 ± 0.17 - | 5.78 ± 0.08 | 100 |
| 3 | 100 | 14.2 | 0.58 (+) | 12.6 | 1.3 ± 0.15 - | 5.75 ± 0.09 | 100 |
| Leaf explant | |||||||
| 0.5 | 70 | 29 | 0.52 (+) | - | - | - | - |
| 1 | 70 | 29.1 | 0.54 (+) | - | - | - | - |
| 1.5 | 80 | 28.5 | 0.55 (+) | - | - | - | - |
| 2 | 90 | 28.9 | 0.60 (++) | - | - | - | - |
| 2.5 | 100 | 27.5 | 0.63 (++) | - | - | - | - |
| 3 | 100 | 28.9 | 0.65 (++) | - | - | - | - |
Note: +=Slight callus, ++=Medium callus, R+= Slight roots, R+++=Profuse roots.
Table 4: The morphogenetic impact of varied auxin (NAA) concentrations supplied separately in the medium (MS) on distinct explants, such as the nodal segments, shoot apexes, and leaves.
During the first 10 to 12 days of incubation, leaf explants cultured on MS media supplemented with different concentrations of NAA showed an increase in leaf size. Within 26-30 days of incubation with 70–100% frequency, induction of light brown callus was seen from the cut ends of leaf explants at all NAA levels (0.5-3.0 mg/l). 3.0 mg/l NAA produced the largest callus weight (0.65 g), which was followed by 2.5 mg/l (0.63 g) with 100% frequency (Table 4).
Impact of combined additions of cytokinins (BAP/Kn) and auxins (IAA/NAA) to the basal medium
When added together in MS media, cytokinins and auxins either caused shoot buds/callus/roots in the nodal segment, shoot apex, and leaf explants.
Impact of auxin (NAA)+cytokinin (BAP): In nodal segment explants, (1.0 and 2.0 mg/l) NAA and (1.0 and 2.0 mg/l) BAP together produced only shoot buds with 100% frequency within 14-16 days of incubation. Induction of the highest shoot buds (1.6) was noted at 1.0 mg/l BAP+1.0 mg/l NAA (Table 5 and Figure 7).
| Concentration (mg/l) | Response (%) | Callus | Shoot multiplication | ||||
|---|---|---|---|---|---|---|---|
| Days taken for callus initiation | Fresh callus weight (g) | Days taken for sprouting | Number of shoot buds/explant | Shoot length (cm) | Response (%) | ||
| Nodal segment explant | |||||||
| BAP | NAA (1.0 mg/l) | ||||||
| 1 | - | - | - | 15.4 | 1.6 ± 0.16 | 6.15 ± 0.06 | 100 |
| 2 | - | - | - | 15.3 | 1.5 ± 0.17 | 6.20 ± 0.03 | 100 |
| BAP | NAA (2.0 mg/l) | ||||||
| 1 | - | - | - | 14.4 | 1.3 ± 0.15 | 5.95 ± 0.08 | 100 |
| 2 | - | - | - | 14.4 | 1.4 ± 0.16 | 6.08 ± 0.09 | 100 |
| Shoot apex explant | |||||||
| BAP | NAA (1.0 mg/l) | ||||||
| 1 | 100 | 14.5 | 0.62 (++) | 14 | 1.7 ± 0.15 R+ | 6.01 ± 0.09 | 100 |
| 2 | 100 | 13.9 | 0.63 (++) | 14.6 | 1.6 ± 0.16 R+ | 5.92 ± 0.08 | 100 |
| BAP | NAA (2.0 mg/l) | ||||||
| 1 | 100 | 13.3 | 0.60 (++) | 14.3 | 1.4 ± 0.16 | 5.85 ± 0.09 | 100 |
| 2 | 100 | 14.5 | 0.58 (+) | 16.3 | 1.3 ± 0.15 | 5.72 ± 0.14 | 100 |
| Leaf explant | |||||||
| BAP | NAA (1.0 mg/l) | ||||||
| 1 | 100 | 23.9 | 0.85 (+++) | - | - | - | - |
| 2 | 100 | 23.6 | 0.59 (+) | - | - | - | - |
| BAP | NAA (2.0 mg/l) | ||||||
| 1 | 100 | 23.4 | 0.60 (++) | - | - | - | - |
| 2 | 100 | 26.8 | 0.63 (++) | - | - | - | - |
Note: +++=Profuse callus, ++=Medium callus, +=Slight callus, R+= Slight roots.
Table 5: The morphogenetic impact of varied concentrations of auxin (NAA) and cytokinin (BAP) added together in the MS medium on distinct explants, such as the nodal segment, shoot apex, and leaf.
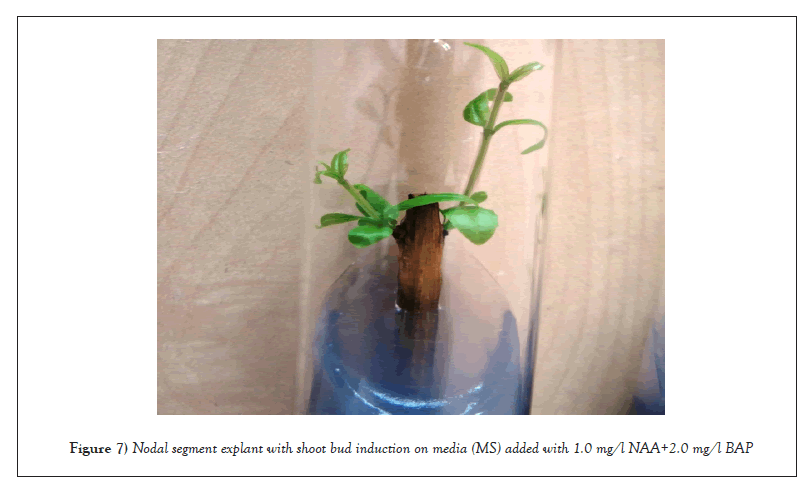
Figure 7: Nodal segment explant with shoot bud induction on media (MS) added with 1.0 mg/l NAA+2.0 mg/l BAP.
In 14-16 days and 14-17 days, respectively, the injection of a shoot apex explant on medium supplemented with a mixture of BAP (1.0 mg/l and 2.0 mg/l) and NAA (1.0 mg/l and 2.0 mg/l) produced a shoot bud and callus. The leaf explant did not respond for a considerable amount of time. After 23-27 days of incubation at BAP (1.0 mg/l and 2.0 mg/l)+NAA (1.0 mg/l and 2.0 mg/l), a later semi-compact, greenish yellow callus began to form from the cut surface of the explants. At 100% frequency, the greatest callus (0.85 g) proliferation was recorded at 1.0 mg/l NAA+1.0 mg/l BAP. (Figure 8). During the cultural process, the semi-compact greenish yellow callus that was created at the ends of the leaf explants was powerful and utilized for shoot morphogenesis (Table 5).

Figure 8: Callus formation in a leaf explant added with BAP @ 1.0 mg/l and NAA @ 1.0 mg/l on MS media.
Impact of auxin (IAA)+cytokinin (BAP): Only shoot buds were induced in nodal segment explants within 15-17 days of inoculation with 100% frequency for all combinations of BAP (1.0 mg/l and 2.0 mg/l) and IAA (1.0 mg/l and 2.0 mg/l). While the largest shoot bud (1.7) was found at 1.0 mg/l BAP+2.0 mg/l IAA, the longest shoot length (6.21 cm) was found at 1.0 mg/l BAP+1.0 mg/l IAA. BAP and IAA alone were not enough to cause a callus to form in nodal segment explants (Table 6). The first induction of shoot buds was brought about by the interaction between BAP (1.0 mg/l and 2.0 mg/l) and IAA (1.0 and 2.0 mg/l), and later on, a slight to medium callus separated from the base of the shoot explants. After the vaccination, callus induction was initiated within 15-17 days. At 1.0 mg/l BAP+2.0 mg/l IAA, the highest callus weight (0.65 g) was recorded. On the other hand, shoot bud induction began in shoot apex explants within 14 to 15 days of inoculation at all levels of combinations. At 2.0 mg/l BAP+1.0 mg/l IAA, the maximum shoot length and highest shoot bud induction (1.8) were noted. Additionally, little roots were seen in every combination (Table 2).
| Concentration (mg/l) | Response (%) | Callus | Shoot multiplication | ||||
|---|---|---|---|---|---|---|---|
| Days (taken) for callus initiation | Callus (Fresh) weight (g) | Days (taken) for sprouting | Number of shoot buds /explant | Shoot length (cm) | Response (%) | ||
| Nodal segment explant | |||||||
| BAP | IAA (1.0 mg/l) | ||||||
| 1 | - | - | - | 17 | 1.3 ± 0.15 | 6.21 ± 0.03 | 100 |
| 2 | - | - | - | 15.2 | 1.5 ± 0.17 | 5.80 ± 0.09 | 100 |
| BAP | IAA (2.0 mg/l) | ||||||
| 1 | - | - | - | 16.4 | 1.7 ± 0.15 | 5.94 ± 0.07 | 100 |
| 2 | - | - | - | 16 | 1.3 ± 0.15 | 5.62 ± 0.06 | 100 |
| Shoot apex explant | |||||||
| BAP | IAA (1.0 mg/l) | ||||||
| 1 | 100 | 16.5 | 0.59 (+) | 15.3 | 1.7 ± 0.15 R+ | 5.53 ± 0.07 | 100 |
| 2 | 100 | 15.1 | 0.57 (+) | 14 | 1.8 ± 0.13 R+ | 6.21 ± 0.07 | 100 |
| BAP | IAA (2.0 mg/l) | ||||||
| 1 | 100 | 16.1 | 0.65 (++) | 14.8 | 1.6 ± 0.16 R+ | 5.55 ± 0.08 | 100 |
| 2 | 100 | 15.1 | 0.56 (+) | 14.5 | 1.4 ± 0.16 R+ | 5.50 ± 0.06 | 100 |
| Leaf explant | |||||||
| BAP | IAA (1.0 mg/l) | ||||||
| 1 | 100 | 25.7 | 0.70 (+++) | - | - | - | - |
| 2 | 100 | 25.2 | 0.73 (+++) | - | - | - | - |
| BAP | IAA (2.0 mg/l) | ||||||
| 1 | 100 | 27 | 0.62 (++) | - | - | - | - |
| 2 | 100 | 26.8 | 0.65 (++) | - | - | - | - |
Note: +++=Profuse callus, ++=Medium callus, +=Slight callus, R+= Slight roots.
Table 6: The morphogenetic impact of varied concentrations of auxin (IAA) and cytokinin (BAP) added together in the medium (MS) on distinct explants, such as the nodal segments, shoot apexes, and leaves.
Within 25 to 27 days of the leaf explant being infected in MS media supplemented with BAP and IAA, reddish brown callus induction began on the cut ends of the explant. In leaf explants, every combination level caused callus with 100% frequency. Induction of callus was highest (0.73) at 2.0 mg/l BAP+1.0 mg/l IAA.
Effects of auxin (NAA) and cytokinin (Kn): For the purpose of inducing shoot buds, nodal segment explants were inoculated in Kn (1.0 mg/l and 2.0 mg/l) enriched medium (1.0 mg/l and 2.0 mg/l). Within 14–17 days following inoculation, 100% of the time, shoot bud induction began. At 1.0 mg/l Kn+2.0 mg/l NAA, the highest shoot length (6.09 cm) and highest shoot bud (1.7) induction were noted. Kn and NAA alone were insufficient to cause callus formation in nodal segment explants (Table 7). In response to combined Kn (1.0 mg/l and 2.0 mg/l) and NAA (1.0 mg/l and 2.0 mg/l) treatments, shoot apex explants generally showed callus and shoot bud induction. Within 15 to 17 days of the inoculation, 100% frequency callus induction was initiated at all combination levels. Induction of the highest callus (0.65 g) was noted at 2.0 mg/l Kn+2.0 mg/l NAA. After inoculation, shoot bud induction was initiated 15-17 days later with 100% frequency. The combination of 1.0 mg/l Kn+2.0 mg/l NAA produced the highest shoot bud induction (1.6) and the longest shoot length (6.01) at 1.0 mg/l Kn+1.0 mg/l NAA. Additionally, little roots were seen in every combination (Table 7).
| Concentration (mg/l) | Response (%) | Callus | Shoot multiplication | ||||
|---|---|---|---|---|---|---|---|
| Days (taken) for callus initiation | Callus (Fresh) weight (g) | Days (taken) for sprouting | Number of shoot buds /explant | Shoot length (cm) | Response (%) | ||
| Nodal segment explant | |||||||
| Kn | NAA (1.0 mg/l) | ||||||
| 1 | - | - | - | 16.5 | 1.5 ± 0.17 | 5.67 ± 0.16 | 100 |
| 2 | - | - | - | 14.8 | 1.4 ± 0.16 | 5.68 ± 0.15 | 100 |
| Kn | NAA (2.0 mg/l) | ||||||
| 1 | - | - | - | 14.9 | 1.7 ± 0.15 | 6.09 ± 0.21 | 100 |
| 2 | - | - | - | 15.5 | 1.4 ± 0.16 | 5.97 ± 0.16 | 100 |
| Shoot apex explant | |||||||
| Kn | NAA (1.0 mg/l) | ||||||
| 1 | 100 | 16.5 | 0.57 (+) | 16.3 | 1.5 ± 0.17 R+ | 6.01 ± 0.17 | 100 |
| 2 | 100 | 15.1 | 0.57 (+) | 16.6 | 1.4 ± 0.16 R+ | 5.63 ± 0.13 | 100 |
| Kn | NAA (2.0 mg/l) | ||||||
| 1 | 100 | 16.1 | 0.59 (+) | 15.6 | 1.6 ± 0.16 R+ | 5.87 ± 0.11 | 100 |
| 2 | 100 | 15.1 | 0.65 (++) | 16 | 1.5 ± 0.17 - | 5.37 ± 0.27 | 100 |
| Leaf explant | |||||||
| Kn | NAA (1.0 mg/l) | ||||||
| 1 | 100 | 25.1 | 0.57 (+) | - | - | - | - |
| 2 | 100 | 25 | 0.60 (++) | - | - | - | - |
| Kn | NAA (2.0 mg/l) | ||||||
| 1 | 100 | 27 | 0.58 (+) | - | - | - | - |
| 2 | 100 | 26 | 0.53 (+) | - | - | - | - |
Note: +=Slight callus, ++=Medium callus, R+= Slight roots.
Table 7: Effect of varying concentrations of cytokinin (Kn) and auxin (NAA) combined with medium (MS) on the morphology of diverse explants, including nodal segments, shoot apexes, and leaves.
After 25 to 27 days of inoculation, the morphogenetic response in leaf explants cultured on Kn+NAA supplemented media began to take the shape of compact reddish green callus induction on its cut ends. Every combination level exhibits 100% frequency of callus induction. Induction of callus (0.60 g) was highest at 2.0 mg/l Kn+1.0 mg/l NAA.
Impact of auxin (IAA)+cytokinin (Kn): Kn (1.0 mg/l and 2.0 mg/l) and IAA (1.0 mg/l and 2.0 mg/l) supplements were added to MS medium for the incubation of nodal explants. After the inoculation, shoot induction was initiated 14–17 days later with 100% frequency. The longest shoot length (6.05 cm) was recorded at 1.0 mg/l Kn+1.0 mg/l IAA, whereas the higher shoot bud (1.7) was noted at 2.0 mg/l Kn and 1.0 mg/l IAA. In shoot apex explants, Kn (1.0 mg/l and 2.0 mg/l) and IAA (1.0 mg/l and 2.0 mg/l) together produced callus induction and shoot buds. Within 13 to 17 days of the inoculation, callus induction was initiated at every combination level with 100% frequency. The callus with the highest value (0.63) was found with 1.0 mg/l Kn+2.0 mg/l IAA. On the other hand, shoot bud induction began at all levels of combinations with 100% frequency within 15 to 18 days of inoculation. At 1.0 mg/l Kn+2.0 mg/l IAA, there was a higher shoot bud induction (1.7), and at 2.0 mg/l Kn+1.0 mg/l IAA, there was the greatest shoot length (6.02). The measured medium roots were 2.0 mg/l Kn+1.0 mg/l IAA (Table 8).
| Concentration (mg/l) | Response (%) | Callus | Shoot multiplication | ||||
|---|---|---|---|---|---|---|---|
| Days (taken) for callus initiation | Callus (Fresh) weight (g) | Days (taken) for sprouting | Number of shoot buds /explant | Shoot length (cm) | Response (%) | ||
| Nodal segment explant | |||||||
| Kn | IAA (1.0 mg/l) | ||||||
| 1 | - | - | - | 14.5 | 1.4 ± 0.16 | 6.05 ± 0.14 | 100 |
| 2 | - | - | - | 16.5 | 1.7 ± 0.15 | 5.79 ± 0.14 | 100 |
| Kn | IAA (2.0 mg/l) | ||||||
| 1 | - | - | - | 15.8 | 1.5 ± 0.17 | 5.92 ± 0.17 | 100 |
| 2 | - | - | - | 15.4 | 1.5 ± 0.17 | 5.85 ± 0.15 | 100 |
| Shoot apex explant | |||||||
| Kn | IAA (1.0 mg/l) | ||||||
| 1 | 100 | 16.1 | 0.61 (+) | 15.5 | 1.4 ± 0.16 R+ | 5.85 ± 0.24 | 100 |
| 2 | 100 | 14.9 | 0.58 (+) | 17.8 | 1.4 ± 0.16 R++ | 6.02 ± 0.13 | 100 |
| Kn | IAA (2.0 mg/l) | ||||||
| 1 | 100 | 13.6 | 0.63 (+) | 17.8 | 1.7 ± 0.15 R+ | 6.01 ± 0.15 | 100 |
| 2 | 100 | 14.4 | 0.57 (++) | 15.2 | 1.5 ± 0.17 - | 5.94 ± 0.13 | 100 |
| Leaf explant | |||||||
| Kn | IAA (1.0 mg/l) | ||||||
| 1 | 100 | 25 | 0.67 (++) | - | - | - | - |
| 2 | 100 | 25.9 | 0.58 (++) | - | - | - | - |
| Kn | IAA (2.0 mg/l) | ||||||
| 1 | 100 | 24.8 | 0.57 (+) | - | - | - | - |
| 2 | 100 | 24.9 | 0.58 (+) | - | - | - | - |
Note: +=Slight callus, ++=Medium callus, R+= Slight roots, R++= Medium roots.
Table 8: The morphogenetic impact of varying quantities of auxin (IAA) and cytokinin (Kn) added together in the medium (MS) on distinct explants, such as the nodal segment, shoot apex, and leaf.
Within 24 to 26 days of inoculation, leaf explants cultured on MS medium supplemented with Kn (1.0 mg/l and 2.0 mg/l)+IAA (1.0 mg/l and 2.0 mg/l) showed semi-compact mild green callus induction at 100% frequency across all combination levels. A callus induction of the highest degree (0.67) was seen at 1.0 mg/l Kn+1.0 mg/l IAA.
Effect of PGRs
After the discovery of kenetin, the theory that morphogenesis might be controlled chemically was further developed. Skoog and Miller [7] postulated that the auxin:cytokinin ratio regulates development in cultured tissues. Numerous discrepancies have been noted in the hypothesis' testing using huge numbers of cell and tissue cultures. It is now widely known that a wide range of additional conditions alter or completely cancel the auxin:cytokinin ratio's response.
The impact of cytokinins (when added individually to the media)
Cytokinins have been shown to have the most notable effect on bud breaks and shoot multiplication [8]. The two cytokinins most frequently utilized for micro propagation are BAP and Kn. In the current study, both cytokinins stimulated shoot bud at all levels in the nodal segment and shoot apex explants when added separately to the basal media. When shoot apex and nodal segment explants were injected on basal medium containing 2.0 and 2.5 mg/l BAP, respectively, the highest level of shoot bud induction was seen.
These findings about plant growth regulator (BAP) were consistent with those of Kumari [9] and Gupta et al., [10]. In Bauhinia variegate, she saw the highest rate of shoot multiplication at 5.0 mg/l BAP. Nonetheless, in the current investigation, the highest rate of shoot proliferation was noted at 2.0 and 2.5 mg/l BAP. There could be a difference in genus causing this variation.
Ali et al., [11] in guava, Widiyanto et al., [12] in teak, Golozan and Shekatendeh [13] in Punica granatum, Kumar and Singh [14] in Prosopis, Borthakur et al., [15] in Albizza chinesis, Choudhari et al., [16] in jamun, and Bensaad and Milad, [17] in Punica granatum have also noted the role of BAP for shoot induction. These outcomes closely matched those of the current investigation. The fact that the BAP supplementation range was 0.5-10.0 mg/l, however, may have been caused by the use of various explant types and genera.
The literature contains full of instances where cytokinins have been shown to be useful in causing several shoots to emerge from apical/axillary explants, presumably as a result of pre-existing meristems. In actuality, the species has fully utilized this characteristic for micro propagation [1]. Certain explants, such as the cotyledonary segment of soybean [18,19], the hypocotyls and cotyledon explants of V. aconitifolia [20], and the leaf and stem explants of Cicer arietinum L. [21] have all been shown to induce multiple shoots in addition to shoot apicals/axillaries. Further, Tables 1 and 2 revealed that within 15 to 28 days of incubation, both the leaf and shoot apex explants under the effect of BAP and Kn showed mild to profuse callus induction. Under BAP, callus differentiation was greater than in media supplemented with Kn. The outcomes align with those of Omura et al., [22], who observed a significant incidence of callus formation in dwarf pomegranate leaf segments at 5 μM BAP and in fenugreek shoot apex explants [23].
Auxins' (single-incorporated in the medium) effects
The main uses of auxins are initiation of callus and rooting. PGRs in the medium and the composition of the basal salt were said to control the shoot's ability to root [24-27]. Auxin is necessary for all most species in order to initiate rooting. For root induction, the most popular agents are IBA and NAA [24]. In vitro root induction was achieved by using the IBA in a variety of plants, including Carnation [28] Hydichium roxburgii [29], and Lycopersicon esculentum [30]. In contrast to cytokinins, auxins significantly varied the responses that several pomegranate explants elicited in the current study. In the nodal segment, auxins (IBA/NAA) produced shoot bud and roots; in the shoot apex and leaf explants, they induced shoot apex and callus. Fougat et al., [31] also reported callus induction in pomegranate cotyledon and leaf explants. The current study also found that adding auxins or auxin and cytikinin together to MS media resulted in root induction. Similar findings were obtained by Helaly et al., [32] in the case of pomegranates, Naik et al., [33], and Ali et al., [11] in the case of guavas. Auxin-supplemented media caused root induction in pomegranates, according to their observations.
Interaction between auxins and cytokinins
When Skoog [34,35], Skoog and Tsui [36] and Skoog and Miller [7] showed that A methodical approach to organogenesis in vitro was started since it was discovered that it was possible to more or less promote shoot/root differentiation in tobacco by adjusting the ratio of IAA and adenine/Kinetin in the culture media. Skoog and Miller [7] rejected Went's [37] theory that certain substances cause the development of specific organs. Instead, they held that ratios, or quantitative interactions, rather than absolute concentrations of substances involved in development and growth, determine the formation of organs. While the opposite situation favors the induction of shoot buds, a high level of auxin to cytokinin promotes the growth of roots. For many species, the so-called Skoog-Miller model is reliable. In the current study, auxins and cytokinin addition resulted in the induction of shoot buds, calluses, and roots in the explants. In leaf explants, profuse callus was seen with 100% frequency at 1.0 mg/l NAA+1.0 mg/l BAP (Table 5 and Figure 8). Murkute et al., [38] have documented a noteworthy function of NAA and BAP together for the differentiation of callus in cotyledon and pomegranate leaf explant. The combination of cytokinin (BAP/Kn) and auxins (IAA/NAA) supplemented the media, fully inhibiting callus proliferation on the base of nodal segment explants. Thirupathy et al., [39] found that Tefrosia hookeriana did not induce callus from leaves, nodes and internodes explant in MS medium supplemented with 2.0 mg/l 2, 4-D+0.25 mg/l BAP. These discoveries run counter to their findings. This could be because different species and types of explants were employed in this specific study. Similarly, in pomegranate cv. Ganesh, Fougat et al., [31] noted induction of callus from cotyledons and leaves explant on media (MS) supplemented with 2.0 mg/l Kn+4.0 mg/l NAA. In 2005, Jarzina and colleagues [40] documented the formation of callus in leaf explants from five distinct hemp types grown on MS media supplemented with 1.0 mg/l Kn+0.5 mg/l NAA. The results were in conflict with the current findings due to variations in the taxa, species, and kind of explant utilized in the investigation. Multiple shoots were induced in shoot apex and nodal segment explants in response to the interaction effects of cytokinin and auxins. Jaidka and Mehra [41], Mukrute et al., [38] and Chaugule et al., [42] have noted similar outcomes in pomegranates by documenting the function of NAA+BAP in shoot bud induction from shoot tip, nodal segments and cotyledon. In several plant species, it has been found that combinations of auxins and cytokinins can stimulate shoot organogenesis from a variety of explants. Convolvulus single-cell tissue clones produced shoot buds even in the absence of growth regulators; yet, IAA and kinetin exhibited the highest frequency of bud differentiation [43]. De novo shoot regeneration was obtained in the current study using a medium supplemented with BAP@ 2.0 mg/l or BAP@1.0 mg/l+NAA@2.0 mg/l at a frequency of 60%. In 1981, Kartha et al., [44] noted that BAP and NAA together caused numerous shoot initiation in Arachis hypogea. Also obtained were entire plants. Multiple shoot buds multiplied in Cyamopsis tetragonoloba shoot tip explants on a medium enriched with BAP with IAA/IBA [45]. Inducing adventitious shoots from callused cotyledonry tissues of H. tuberosum was found to be particularly successful with a combination of BAP (0.1 mg/l) and NAA (0.5 mg/l) [46]. According to the previous explanation, cytokinin and auxin have an interaction impact that stimulates shoot/root organogenesis; however, this effect differs depending on the species and kind of explant. While virtually every portion of some plants, like tobacco, can be altered in vitro, this possibility is limited to specific tissues in other plants [47].
In present research investigation, best media to induce shoot buds in nodal segment explants was found to be MS media supplemented with 2.0 mg/l BAP. The highest degree of shoot bud induction in shoot apex explants was observed with MS media having 2.5 mg/l BAP. Rich callus induction was accomplished in shoot apex explants by utilizing MS medium supplemented with 2.0 mg/l BAP. The best media for inducing callus in leaf explants was found to be MS media supplemented with 1.0 mg/l BAP+1.0 mg/l NAA.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Kumar R. Plant growth regulators' effects on various pomegranate cv. Sindhuri explants cultured in vitro. AGBIR.2023;39(6):723-735.
Received: 01-Nov-2023, Manuscript No. AGBIR-23-122049; , Pre QC No. AGBIR-23-122049 (PQ); Editor assigned: 03-Nov-2023, Pre QC No. AGBIR-23-122049 (PQ); Reviewed: 22-Nov-2023, QC No. AGBIR-23-122049; Revised: 29-Nov-2023, Manuscript No. AGBIR-23-122049 (R); Published: 06-Dec-2023, DOI: 10.35248/0970-1907.23.39.723-735
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
