Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2022) Volume 38, Issue 1
This study was conducted to determine the changes in physiological and some biochemical responses of selected rainfed lowland rice genotypes under drought condition. AL-108, AL-87, AL-97, AL-55 and AL-5, initially selected based on evaluation during germination and seedling stages; and NSIC Rc14 (tolerant check) PSB Rc82 (moderately susceptible check) were evaluated under drought and well-watered condition from 20 DAS and 30 DAS for a period of 12 days. Varying tolerance in terms of physiological and biochemical traits under different period of drought imposition were observed in different genotypes. All of the test genotypes had high photosynthetic efficiency under drought although some biochemical responses differed.Relative water content, stomatal conductance, transpiration rate and photosynthetic rate were reduced in all test genotypes but more severe reduction was observed in susceptible check (PSB Rc82). Transpiration rate was minimal in tolerant genotypes (NSIC Rc14). Total chlorophyll and carotenoids decreased under drought in most the genotypes, except for AL-108, AL-5 and AL- 97. Concomitantly, the amount of antioxidant and total soluble sugar in the drought-tolerant genotypes increased markedly during drought stress, while decreased in susceptible variety. It can be concluded that maintenance of relative water content and photosynthetic efficiency, and increase in antioxidant level and total soluble sugar accumulation, were associated with the drought tolerance of the rainfed lowland rice varieties.
Photosynthesis; Stomatal conductance; Relative water content; Relative water content
Drought is considered the most important stress limiting yield in rain fed lowland, and responsible for the seasonal fluctuations in rice growth and development. Drought is estimated to frequently affect around 19 to 23 M ha. Growth and performance of a rice genotype under drought condition is usually reflected on its physiological, agronomic, and potential yield response. These are commonly observed in rice production systems, wherein grain yield gap between drought-prone areas (such as upland and rainfed lowland) and irrigated lowlands exists. For instance, irrigated lowlands have an average productivity of 6–8 t ha-1 while only about 1 t ha-1 in rain fed areas planted with rain fed adapted varieties [1].
Knowledge on the effect of water stress in rice is important since it requires enormous amount of water to produce grain. Rice transpired 500-1000 liters of water to produce 1 kg of rice grains. Due to abnormal occurrences of water scarcity brought about by climate change, continuous improvement of rice to towards drought-tolerance is current focus of rice breeders. Physiological, morphological, and biochemical responses under drought regimes are useful parameters as input for crop improvement. Plant responses to drought stress are complex and involved changes in their morphology, physiology and metabolism [2]. Drought affects the turgor pressure which leads to decrease in the cell growth, cell expansion and cell enlargement. Drought is known to cause great reduction in fresh and dry weight of leaf and shoot. Physiological drought can affect respiration, growth hormone levels, nutrient metabolism, and ultimately photosynthesis and related pigments such as chlorophyll and carotenoid content (Figure 1). On the other hand, total soluble sugar in rice is enhanced after plants are exposed to water stress. Previous findings suggested that soluble sugars are considered compatible solutes that can act as osmo-protectants [3].
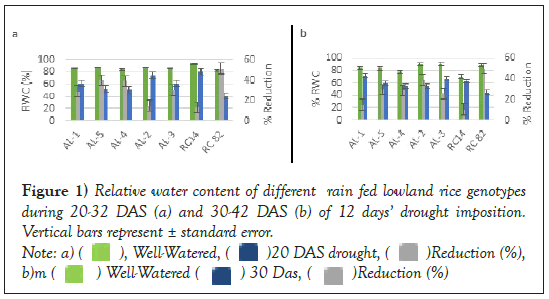
Figure 1: Relative water content of different rain fed lowland rice genotypes during 20-32 DAS (a) and 30-42 DAS (b) of 12 days’ drought imposition. Vertical bars represent ± standard error.

Screening for drought tolerance in rice at seedling stage has been reported. For effective selection of drought tolerant varieties, it is necessary to identify drought tolerant from susceptible genotypes at early vegetative stage. Hence, this study was conducted determine some physiological and biochemical responses in selected rain fed lowland rice genotypes grown under drought stress conditions (Table 1) [4].
| Sources of variation | DF | Means | |
|---|---|---|---|
| Relative water content | |||
| 20 DAS | 30 DAS | ||
| Treatment (T) | 1 | 8347.9696** | 6581.1885** |
| Genotypes (G) | 6 | 371.6040ns | 220.8268ns |
| GxT | 6 | 160.0771ns | 197.3037ns |
| Error | 42 | 434.62 | 402.5921 |
| Mean | 73.79189 | 72.67442 | |
| CV | 28.25181 | 27.60903 | |
Note: *,**, ns, Level of significance *p<0.05; **p<0.01
Table 1 : Analysis of variance, drought at mean squares in relative water content
Greenhouse pot experiment
Five tolerant rain fed rice genotypes: AL-108, AL-87, AL-97, AL-55, AL-5 selected from PEG-induced drought condition and 2 check varieties: NSIC Rc14 (tolerant) and PSB Rc82 (susceptible) were used (Table 2). PSB Rc82 is an irrigated lowland variety and used as susceptible check under rain fed lowland condition in fields trials conducted at University of the Philippines Los Baños [5].
| Source of variation | DF | Mean squares | |
|---|---|---|---|
| Stomatal conductance | |||
| 20 DAS | 30 DAS | ||
| Treatment (T) | 1 | 0.394** | 0.257** |
| Genotypes (G) | 6 | 0.006** | 0.007** |
| GxT | 6 | 0.008ns | 0.012** |
| Error | 42 | 0.003 | 0.003 |
| Mean | 0.159 | 0.149 | |
| CV | 36.175 | 34.499 | |
Note: *,**, ns, Level of significance *p<0.05; **p<0.01
Table 2 : Analysis of variance, drought at mean squares in stomatal conductance
This experiment was conducted under greenhouse condition at the International Rice Research Institute (IRRI), Los Baños, (14 °11’ N, 120 ° 15’ E, 21 masl) from January to April 2019. The soil used was obtained from IRRI upland site, classified as clay loam (30% sand, 38% silt and 32% clay), with 6.2 pH, 0.26% N, 153 ppm P, 5.38 cmolc/kg K (Figure 2).
Figure 2: Stomatal conductance of different rainfed lowland rice genotypes during 20-32 DAS (a) and 30-42 DAS (b) of 12 days’ drought imposition. Vertical bars represent ± standard error.

Two-liter PVC pots filled with 2 kg soil were used as experimental units. Four pre-germinated rice seeds were sown per pot at 0.25-inch sowing depth. The experiment was laid-out in a split-plot, Complete Randomized Design (CRD) with five replications, wherein each pot corresponds to one replicate [6]. Water treatments were designated as main plots, while 7 genotypes as subplots. Drought stress was imposed during 20-32 and 30-42 DAS. Well- watered treatment served as the control. The mean distance between plants was 20 cm. Re-randomization (pot rearrangement) was done every 3 days to maintain homogeneity for light capture and other factors. Pots filled with soil were watered at field capacity at 1 DAS before sowing (Table 3). This condition was maintained until drought stress was induced at 20 and 30 DAS. The control (T0) was well-watered condition (field capacity) that maintained until termination of the experiment. Drought treatment was set to 75% field capacity, while water application was withheld to impose drought at 20-32 DAS (T1) and 30-42 DAS (T2) [7].
| Source of variation | DF | Mean squares | |
|---|---|---|---|
| Transpiration rate | |||
| 20 DAS | 30 DAS | ||
| Treatment (T) | 1 | 89.382** | 92.357** |
| Genotypes (G) | 6 | 1.179** | 1.572** |
| GxT | 6 | 1.956ns | 2.155** |
| Error | 42 | 0.412 | 0.433 |
| Mean | 6 | 2.836 | 3.221 |
| CV | 22.625 | 20.426 | |
Note: *,**, ns, Level of significance *p<0.05; **p<0.01
Table 3 : Analysis of variance, drought at meansquares in transpiration rate
Soil Moisture Content (SMC) was monitored by weighing the pot early in the morning at 2-day interval. SMC was calculated using the following formula:
SMC=Saturated weight of each pot (weight of pot+weight of soil+field capacity)–Current pot weight
In order to achieve the target SMC for all treatments, control pots were watered accordingly with the same amount of water that has been removed through transpiration and root absorption (Figure 3). Drought-treated pots were re-watered when field capacity reaches the lower threshold of 10%. The critical soil moisture for most cereals and legumes is 8% (Suralta and Yamauchi, 2008) [8].
Figure 3: Transpiration rate of different rain fed lowland rice genotypes during 20-32 DAS (a) and 30-42 DAS (b) of 12 days’ drought imposition. Vertical bars represent ± standard error.

Cultural management practices
Top soil (20 cm depth) collected from the upland site of IRRI was used. Weeds were removed and soil was sieved and dried. The air dried soil was placed in a 2-L PVC pot with approximately 2000 g soil in each pot. Seeds from all genotypes were pre-germinated prior to sowing in a petri dish with wet filter paper for 3 days. When roots attained 2 cm, four germinated seeds were sown in moist pot at 2 cm depth. Thinning was done at 10 DAS. The most vigorous seedling/plant was maintained up to the end of the experiment. Urea (46-0-0) was applied at 10 DAS at the rate of 0.3 g per pot. Complete fertilizer (14-14-14) was applied at the rate of 0.3 kg per pot at sowing (Table 4). Weeding was done by manual removal/cleaning every week. Pesticides were applied at recommended rate whenever necessary. For proper seedling growth, pots were watered regularly until imposition of the drought treatment [9].
| Source of variation | DF | Mean squares | |
|---|---|---|---|
| Photosynthetic rate | |||
| 20 DAS | 30 DAS | ||
| Treatment (T) | 1 | 1627.29** | 977.02** |
| Genotypes (G) | 6 | 9.96ns | 24.60* |
| GxT | 6 | 18.42* | 16.63* |
| Error | 42 | 6.99 | 6.81 |
| Mean | 10.62 | 9.93 | |
| CV | 24.88 | 26.27 | |
Note: *,**, ns, Level of significance *p<0.05; **p<0.01
Table 4: Analysis of variance and drought at 20 DAS, drought at 30 DAS of selected genotypes under drought
Photosynthetic pigment determination
Total carotenoid content
The determination of total carotenoid content was based on the procedure. In detail, 50 mg of dried sample was extracted thrice with 5.0 mL petroleum ether: acetone (1:1) solvent. Each extraction was followed by 5 min shaking and centrifugation at 3000 x g for 5 min. The pooled supernatant was transferred to a separator funnel and washed twice with 15 mL distilled. The organic layer was collected and dried using anhydrous sodium sulphate [10]. The resulting solution was then diluted to 15 mL using petroleum ether and the absorbance was measured at 450 nm. Total carotenoid content was calculated using the formula:
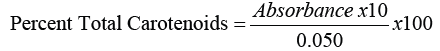
Total chlorophyll content
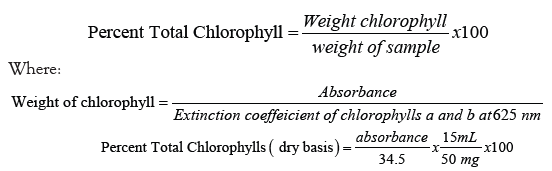
Determination of total chlorophyll content per treatment was done following the method of Yoshida (1972). Fifty (50) milligrams of dried leaf sample was extracted thrice with 4 mL 80% aqueous acetone (v/v). Each extraction was followed by 5 min shaking and centrifugation at 3000 x g for 5 min (Table 5). The supernatant was pooled extract was read at 625 nm wavelength. Chlorophyll was calculated using the formula:
| Drought at 20 DAS | Drought at 30 DAS | ||
|---|---|---|---|
| Well- watered | 25.9895a | Well- watered | 2.0121 a |
| Drought stress | 17.9505b | Drought stress | 1.8027b |
|
|
|||
| AL-55 | 26.68667a | 19.4967 a | |
| AL-87 | 23.330 b | 14.880 f | |
| AL-5 | 20.2133 d | 14.6233 f | |
| AL-108 | 21.8733c | 15.7467c | |
| AL-97 | 19.6867 e | 16.8767 b | |
| Rc 14 | 19.533 e | 15.2667d | |
| Rc 82 | 19.4667 e | 15.0033de | |
| CV | 0.76 | 1.1224 | |
Note: a, b, c, d, e, f, de Values within drought stress treatment with the different letter are significantly different based on comparison using HSD at p ≥0.05 (n = 7).
Table 5 : Analysis of variance and Drought at 20 DAS, Drought at 30 DAS of selected Genotypes under drought
Total antioxidant activity (% RSA)
One gram of dried sample was extracted with 10.0 mL 50% methanol. The resulting mixture was filtered in a clean vial with cover. A 0.10 mL aliquot of the extract was mixed with 4.0 mL distilled water and 1 mL freshly prepared 1 mM dinitrophenyl picryl hydrazyl radical (DPPH) methanolic solution was added. The mixture was left to stand for 30 minutes before reading the absorbance at 517 nm using UV-Vis Spectrophotometer [11]. A blank sample was also read at 517 nm (Table 6). Total antioxidant activity was expressed using Percent Radical Scavenging Activity (% RSA), and computed as:
| Drought at 20 DAS | Drought at 30 DAS | ||
|---|---|---|---|
| Well- watered | 2.6710 a | Well- watered | 2.0121 a |
| Drought stress | 2.2628 b | Drought stress | 1.8027b |
| AL-55 | 3.0302a | 1.9140bc | |
| AL-108 | 2.5557b | 2.0802 a | |
| AL-97 | 2.4543b | 1.9423 b | |
| AL-5 | 2.4087b | 1.6717 e | |
| RC82 | 2.3845bc | 1.7432 d | |
| AL-87 | 2.2588cd | 1.8538 c | |
| RC14 | 2.1762d | 2.1468a | |
| CV | 3.927 | 2.192 | |
Note: a, b, c, d, e, bc, cd Values within drought stress treatment with the different letter are significantly different based on comparison using HSD at p ≥0.05 (n = 7)
Table 6 : Analysis of variance and drought at 20 DAS, drought at 30 DAS of selected genotypes under drought
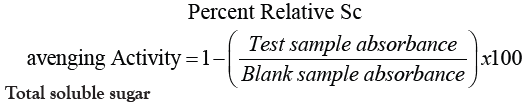
Total soluble sugar
The sample preparation and analysis of total sugar content was a modified procedure from Shallenberg and Birch (1975). Fifty (50) milligrams sample of dried, ground and defatted sample was extracted using 5 mL 80% ethanol twice. The mixture was allowed to stand for 10 min then centrifuged at 3000 rpm for 10 min [12]. The supernatant was separated and added with 0.90 mL distilled water and 3 mL anthrone reagent. The mixture was placed in a boiling water bath for 10 min (Figure 4). The reaction was stopped by placing the container in an ice bath, and allowed to stand until it reached room temperature (Table 7). The resulting mixture was subjected to a colorimetric analysis by reading the absorbance at 630 nm. A standard curve was prepared using glucose as a standard. Percent total soluble sugar was calculated from the standard curve using interpolation method [13].
| Drought at 20 DAS | Drought at 30 DAS | ||
|---|---|---|---|
| Well- watered | 7.6926b | Well- watered | 15.0173a |
| Drought stress | 10.0303 a | Drought stress | 16.6493a |
| Genotypes | |||
| Rc14 | 12.833 a | 15.4393c | |
| AL-97 | 11.8032 a | 12.0453c | |
| AL-87 | 8.4698 ab | 19.1212 b | |
| AL-108 | 8.2425 ab | 19.8032 b | |
| AL-5 | 7.9092 ab | 8.5000d | |
| RC 82 | 6.5603 b | 7.3787d | |
| AL-55 | 6.2120 b | 28.5453 a | |
| CV | 31.81 | 21.55 | |
Note: a, b, c, d, ab, Values within drought stress treatment with the different letter are significantly different based on comparison using HSD at p ≥0.05 (n=7).
Table 7 : Analysis of variance and drought at 20 DAS, drought at 30 DAS of selected genotypes under drought
Figure 4: Relationship between stomatal conductance and transpiration of different rain fed lowland rice genotypes as affected by stress
Physiological measurements
Four replications were measured for the stomatal conductance (gS), photosynthetic rate (PN), and transpiration rate (E) using a Li-Cor 6400 XT at 32 DAS and 42 DAS.
Relative water content (RWC)
Relative water content (RWC) is one of important indicator of leaf water status under drought stress. For each pot, RWC was determined in leaves from middle tiller of three plants, and collected samples were weighed immediately to get the fresh weight. Leaf tissues were rehydrated in water for 24 h until they attained full turgidity, surface-dried and reweighed to get the turgid weight. Finally, the tissues were oven dried at 70°C for 72 h (until constant weight), and were reweighed to obtain the dry weight. RWC was calculated using the following formula [14]:
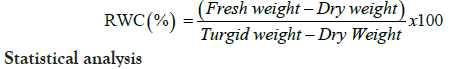
Statistical analysis
Data were analysed with ANOVA, and means were separated by an HSD using
P<0.05. All the analyses were done by using SAS university edition Statistical software.
Drought on physiological characters
Five elite drought tolerant advanced lines along with tolerant check variety NSIC Rc14 and one susceptible check irrigated lowland variety NSIC Rc82 were exposed to 12-day drought and stomatal conductance, net photosynthesis (A), transpiration rate (E), and relative water content were recorded. Results revealed that physiological parameters varied significantly with drought imposition during 20-32 and 30-42 DAS [15].
Relative water content under drought
Relative water content (RWC) is considered the best criterion for monitoring plant water status, as it represents possible variations in water potential, turgor potential, and osmotic adjustment (OA) in plant tissues. RWC reflects the balance between absorbed water by plant and consumed through transpiratio. RWC decreased by 50% in susceptible check (PSB Rc82) and 12% in tolerant check (NSIC Rc14) at 20-32 DAS of stress while 49% in susceptible and 11% in tolerant check at 30-42 DAS during the 12 days of drought. However, RWC did not differ (p<0.05) among the genotypes. With drought imposition, the reduction of RWC in tolerant check was less compared to susceptible genotypes. Among the rice lines, AL-87 and AL-108 had relatively highest RWC when exposed to 12-day drought starting 20-32 DAS and 30-42 DAS, respectively [16].
According to earlier finding, RWC may vary with different rates of stress development and expression of drought responses by different varieties during stress periods. Higher RWC values are recorded in drought stress (Table 8).
| Drought at 20 DAS | Drought at 30 DAS | ||
|---|---|---|---|
| Well- watered | 8.6508 b | Well- watered | 10.5077 b |
| Drought stress | 11.8170 a | Drought stress | 11.4324 a |
| Genotypes | |||
| PUR 55 | 13.5248 a | 9.1207 f | |
| PUR 87 | 11.7958 b | 11.2518 c | |
| PUR 5 | 8.7515 e | 12.8673 a | |
| Rc 82 | 8.2423 e | 10.0755 e | |
| Rc 14 | 9.2193 d | 11.0082 d | |
| PUR 108 | 11.1102 c | 10.9373 d | |
| PUR 97 | 9.2930 d | 11.5295 b | |
| CV | 2.38 | 1.02 | |
Note: a, b, c, d, f Values within drought stress treatment with the different letter are significantly different based on comparison using HSD at p ≥0.05 (n = 7).
Table 8 : Analysis of variance and drought at 20 DAS, drought at 30 DAS of selected genotypes under drought
Tolerant rice genotypes relative to susceptible genotypes under water stress conditions. Reported that RWC of plant exposed to drought stress can decrease to an extent of 60–80% with an increase in osmotic potential of the plant cells. This increases the level of osmolytes and ensures the plant to maintain its water content during drought enabling the plant to sustain its growth and yield (Figure 5). Leaf water use efficiency may be positively correlated with yield when water is a limiting factor for crop growth [17].
Figure 5: Transpiration rate of different rain fed lowland rice genotypes during 20-32 DAS (a) and 30-42 DAS (b) of 12 days’ drought imposition. Vertical bars represent ± standard error.

Based on the results of the present study, it can be concluded that selected tolerant genotypes from PEG drought-induced experiment consistently showed drought tolerance in terms of RWC under greenhouse set-up.
Stomatal conductance under drought
Stomatal conductance indicated the degree of exchange of CO2 and water vapor between ambient and inner leaf. Stomatal conductance decreased with drought imposition at 20-32 DAS and 30-42 DAS in all genotypes relative to the well-watered. Genotypic differences (P>0.05) in stomatal conductance was observed during 30 DAS under well-watered condition, although varying responses to drought were different. The stomatal conductance of susceptible genotype (PSB Rc82) was relatively higher compared with tolerance check NSIC Rc14 under well-watered condition, but was significantly reduced when exposed to drought in both 20 DAS and 30 DAS under 12-day drought imposition. The reduction in stomatal conductance under drought condition might be due to lower leaf relative water content under drought condition (Figure 6). Reported in soybean that reduction in stomatal conductance of drought stressed soybean plants resulted to the loss of leaf turgor and consequent reduction in photosynthetic rate [18].
Figure 6: Effect of drought stress treatment on total chlorophyll content in rice genotype imposed during 20-32 DAS and 30-42 DAS. Vertical bars represent ± standard error.

Among the rice lines, AL-87 had the least reduction in stomatal conductance when drought was imposed during 20 DAS; while in AL-97 in 30 DAS of drought has less reduction with significantly higher when compared with check genotypes. Our results revealed that responses of genotypes to stomatal conductance may vary between genotypes but regardless of which genotypes are higher they were affected when exposed to drought stress. This agrees with the findings that as soil water decreased, stomata conductance, leaf and stem water potential mesophyll conductance to C02 also decreased [19].
On the other hand, pointed out that low stomatal conductance in drought tolerance rice is commonly due to low stomatal density. Reported that stomatal conductance is the complementary selection criteria for stress tolerance of crops. Reduction in stomatal conductance under drought can reduce excessive water loss via transpiration hence maintenance of leaf water potential (Table 9).
| Source of variation | Mean squares | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DF | Total chlorophyll | Total carotenoids | Total antioxidants activity | Total soluble sugar | |||||
| 20 | 30 DAS | 20 | 30 | 20 DAS | 30 | 20 | 30 DAS | ||
| DAS | DAS | DAS | DAS | DAS | |||||
| Treatment (T) | 1 | 1.75** | 0.46** | 678.58** | 239.43** | 57.38** | 27.97 | 105.26** | 8.98** |
| Genotypes (G) | 6 | 0.46** | 0.17** | 53.03** | 17.74** | 38.19** | 327.93** | 23.10** | 8.22** |
| GxT | 6 | 0.41** | 0.45** | 79.87** | 32.35** | 47.54** | 40.53 | 4.83** | 7.94** |
| Error | 42 | 0.01 | 0 | 0.03 | 0.03 | 7.95 | 11.64 | 0.06 | 0.01 |
| Mean | 2.47 | 1.91 | 21.97 | 15.98 | 8.86 | 15.83 | 10.23 | 10.97 | |
| CV | 3.93 | 2.19 | 0.76 | 1.12 | 31.81 | 21.55 | 2.38 | 1.02 | |
Note: *,**,Level of significance *p<0.05; **p<0.01
Table 9 : Analysis of variance and mean performance on total chlorophyll, total carotenoids, total antioxidant activity and total soluble sugar of selected rain fed lowland rice lines under drought
Transpiration rate under drought
Transpiration rate significantly decreased under drought condition regardless of genotypes, a similar trend with stomatal conductance. Significant differences in genotypes were observed at 30-42 DAS imposition of drought. Susceptible check PSB Rc82 had relatively higher transpiration rate compared with resistant check NSIC Rc14 genotype, although not statistically different. Among rice genotypes AL-55 had the highest transpiration rate under well- watered condition but did not differ with the other rice genotypes within 30-24 DAS (Figure 7). It is interesting to note however that AL-87 and AL-97 had the least reduction in transpiration rate among rice lines [20].
Figure 7: Effect of drought stress treatment on total carotenoids content in rice genotypes imposed during 20-32 DAS and 30-42 DAS. Vertical bars represent ± standard error.

The transpiration rate was affected by stomatal conductance wherein transpiration rate increased linearly with stomatal conductance (R2=0.77). Several researches had reported reduction in transpiration rate under drought condition and such response is considered as a drought avoidance mechanism in some rice genotypes, and French bean Islam et al., which are mainly attributed to reduction in stomatal conductance. Reported that stomatal closure due to short-term humidity changes is best correlated with the actual transpirational flux rate and not the humidity gradient between ambient air and leaf. Avoidance of drought can be realized minimizing water loss through reduction of stomatal conductance or the transpiring leaf surface. Moreover, higher value of water use efficiency at the leaf level resulted from lower rates of transpiration rather than from higher rates of photosynthesis (Figure 8) [21].
Figure 8: Effect of drought stress treatment on total antioxidants content in rice genotypes imposed during 20-32 DAS and 30-42 DAS. Vertical bars represent ± standard error.
The results of this study suggest that the reduction in transpiration rate in genotypes AL-87 and AL-97 might have therefore contributed to the high relative water content of these genotypes.
Photosynthesis under drought
Apparent photosynthesis was significantly affected by drought for all genotypes on both 20-32 and 30-42 DAS drought imposition. Net photosynthesis of the resistant check PSB Rc14 and susceptible check PSB Rc82 did not differ while AL-108 had the highest and AL-55 consistently showed high photosynthetic rate under well-watered condition. Under drought condition (20 and 30 DAS drought imposition) AL-108, AL-55, AL-87 and AL-97 and AL-5 had highest photosynthetic rate compared with tolerant and susceptible checks. Rice plants grown under drought condition had reduced photosynthesis relative to those grown under well-watered condition, and this was observed in the present study. Earlier findings showed that drought stress decrease photosynthetic rate of in crops such as mungbean, soybean.and rice [22]. Reduction in photosynthetic rate due to drought is coupled by reductions in leaf expansion, impaired photosynthetic machinery, and pre-mature leaf senescence which are related to carbon assimilation. Under drought stress, many metabolic processes including photosynthesis are negatively affected. For instance, water deficiency damages basic organization structure, which inhibits carbon assimilation and damages the photosynthetic apparatus. Reported that reduction in stomatal conductance and transpiration rate also reduce the photosynthetic rate in rice (Figure 9). Previous studies have showed that decrease in leaf photosynthesis is usually caused by stomatal limitation under mild to moderate drought conditions, while non-stomatal limitation under severe drought conditions [23-25].
Figure 9: Effect of drought stress treatment on total soluble sugar content in rice genotypes imposed during 20-32 DAS and 30-42 DAS. Vertical bars represent ± standard error.
Results of this study suggest that, the high relative water content, minimal transpiration rate and stomatal conductance might have contributed to maintenance of photosynthesis under drought particularly for genotypes AL- 87 AL1 and AL-97.
Total chlorophyll and carotenoid contents under drought
Chlorophyll and carotenoid content are the major factors affecting photosynthetic capacity. Chlorophyll regulates the photosynthetic potential of plants by capturing light energy from the sun while carotenoids participate in harvesting light energy for photosynthesis. These two pigments are vital in the photosynthetic process in plants.
Effect of drought stress was significant (p<0.01) on total chlorophyll and carotenoids content of genotypes and its interaction. Drought significantly reduced total chlorophyll and carotenoids content regardless of genotypes [26,27]. The observation conforms to the earlier findings that water deficit destroys and inhibit synthesizing of the chlorophyll or carotenoids. Damage to leaf pigments as a result of water deficit was also claimed that reduction in chlorophyll level is considered a symptom of oxidative stress and may be the result of pigment photo-oxidation and chlorophyll degradation. attributed the decrease in chlorophyll and carotenoid content to be consequence of drought stress is brought about by the increased production of reactive oxygen species (ROS) such as O2- and H2O2, which led to lipid peroxidation and consequently destruction of photosynthetic pigments. Based on the result of this study, resistant check has higher chlorophyll content when drought was imposed at 30-42 DAS than at 20-32 DAS, although their total carotenoids were comparable in both stages. Among rice lines, AL-55 had the highest chlorophyll content at 20-32 DAS drought period while AL-108 had the highest chlorophyll content when under drought within 30-42 DAS. Similar trend was observed on total carotenoids, AL-108 and AL-55 had consistently higher total carotenoids contents though AL-108 had lower carotenoid content during 30-42 DAS drought period. Furthermore, AL-55 (20 DAS) and AL-108 (30 DAS) were found to have higher chlorophyll contents during drought compared with the rest of genotypes. These genotypes showing higher photosynthetic pigments, such as chlorophyll and carotenoids, also had consistently high photosynthetic rate [28-30].
The results of this study suggest that higher chlorophyll and carotenoid content can be considered as indicators for drought tolerance, this is consistent with the results of their comparative screening of barley genotypes. They concluded higher chlorophyll content was generally associated with high drought tolerance. Furthermore, results also showed that responses of genotypes may differ depending on time of drought stress imposition. In the present study, imposition of drought at both 20-32 DAS and 30-42 DAS has negative effect on total chlorophyll and carotenoids contents but more severe effect of drought was observed at 30 DAS of drought imposition. Hence, screening during vegetative stage is best to done at 30-42 DAS to determine genotypes with higher tolerance to drought stress in terms of total chlorophyll and total carotenoids response [31-34].
Drought and antioxidants in rice leaves
Antioxidants are well-known organic compounds that help plants to fight against free radicals caused by drought stress. High antioxidant activity is a good indicator of drought tolerance. In this study, the antioxidant activity was measured through DPPH scavenging activity. Results showed significant differences in drought treatment and genotypes under drought within 20-32 DAS, while only variation in genotype response was significant during 30-42 DAS drought period. The total antioxidant of two check varieties increased under drought stress, although NSIC Rc14 has relatively higher total antioxidant scavenging activity than PSB Rc82. During 30-42 DAS drought period, the susceptible check PSB Rc82 also showed reduction in antioxidant activity compared with all the genotypes evaluated, suggesting that, the tested lines showed tolerance in terms of antioxidant activity [35,36]. The result of this study conforms to the findings on the elevated scavenging activity of the tolerant rice genotypes under water stress. Enhanced production of antioxidants in rice plants exposed to environmental stresses, such as drought. Among the lines evaluated, AL-97 obtained the highest (61%) increased in its total antioxidants content in both 20-32 DAS of stress imposition while AL-55 had the highest (58%) total antioxidant in 30-42 DAS.Furthermore, AL-87 also showed increased in antioxidant activity during 30-42 DAS drought which is manifested on low reduction in photosynthetic activity of this line. Hence, increased in total antioxidants in AL-55 and AL-97 genotypes suggest their scavenging ability of the synthesized free radicals that harms plant during drought stress [37-39].
Nevertheless, all antioxidants activity increased regardless of rice lines. However, it appears that specific genotype response varied with the timing of drought imposition. For example, AL-55 showed high antioxidant activity during 30-42 DAS drought period but not during 20-32 DAS [40- 43]. Furthermore, during 30-42 DAS of drought regime, no significant difference was observed on between well-watered and drought condition while there was a relative increase in total antioxidant activity under drought stress[44-46]. The result of this study suggest that tolerance of plant may vary at different stages of seedling, in which at drought occurrence some other sources of tolerance could be expressed and these work together to maintain photosynthetic activity and survival of plants under drought condition[47].
Total soluble sugar
Soluble sugars are important metabolites that sustain plant growth and development especially under various stress condition. Result of the analysis of variance revealed that total soluble sugar was significantly influenced by the interaction effect of drought treatment and rice genotypes. Between check varieties, tolerant check (NSIC Rc14) has significantly increased total soluble sugar in the leaves compared with the susceptible check (PSB Rc82). Among rice lines, AL-55 had the highest amount of total soluble sugar, while highest increase was obtained in AL-108 during 20-32 DAS drought imposition. At 30-42 DAS stress imposition, AL-5 was observed to have the highest amount and increase of total soluble sugar. Total soluble sugar increased under drought during 20-32 DAS drought period regardless of rice lines, while total soluble sugar was found to slightly decrease in some lines such as AL-55 and AL-87 during 30-42 DAS drought. Results of this study suggest that the production of this osmolyte is a common response under drought condition. In an early study it was concluded that soluble sugars contributed about 30%-50% of the osmotic adjustment in the leaves of many glycophytes [48].
The accumulation of soluble sugar in plants during drought stress. It is well-known that soluble sugars have complex role in plant metabolism as by- products of hydrolytic processes, substrates in biosynthetic processes, energy production as well as signal for metabolic regulations [49]. These soluble sugars include fructose, glucose, sucrose and possibly trehalose, which play significant roles as compatible osmolytes for osmoregulation, osmotic adjustment, and maintaining the growth and structure of plant tissues by stabilizing cellular membranes and maintenance of turgor pressure in the cell. The increase in soluble sugar in the leaves of tested genotypes therefore may be considered as a tolerance mechanism in rice particularly for rice lines AL-5 and AL-108 due to increased levels during both period of drought imposition [50].
This study revealed that rice genotypes have varying mechanisms of tolerance to drought stress. For instance, AL-108, AL-87, and AL-55 have high photosynthetic efficiency, while AL-97 has higher antioxidants compared with other genotypes. NSIC Rc14 (tolerant check) has exhibited tolerance traits under drought, based on physiological and biochemical characters (total antioxidant activity and total soluble sugar) than the susceptible check (PSB Rc82). Although photosynthetic pigments were reduced under drought condition, higher photosynthetic efficiency was maintained through higher stomata conductance, low transpiration, and high relative water content in the leaf. PSB Rc82 (susceptible check) might not be considered extremely susceptible to drought because most physiological traits are similar to other tolerant lines, except for total antioxidant activity. Moreover, PSB Rc82 has lower tolerance compared to the selected drought tolerant lines and tolerant check NSIC Rc14. Plants may have different response and defense mechanisms under stress. Some traits might dominate over others but overall, these mechanisms contribute to plant survival under drought stress.
Citation: Gaurana ML. Physiological and biochemical responses of rain fed lowland rice genotypes at vegetative stage to drought stress . AGBIR. 2022;38(1):235- 242
Received: 04-Jan-2022, Manuscript No. AGBIR-22-40376; , Pre QC No. AGBIR-22-40376 (PQ); Editor assigned: 06-Jan-2022, Pre QC No. AGBIR-22-40376 (PQ); Reviewed: 20-Jan-2022, QC No. AGBIR-22-40376; Revised: 25-Jan-2022, Manuscript No. AGBIR-22-40376 (R); Published: 01-Feb-2022, DOI: 10.35248/0970-1907.22.37.235-242
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
