Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2024) Volume 40, Issue 2
Greenhouse-grown crops are generally considered safer than open field crops. The study objectives were to determine whether greenhouse-grown crops in Trinidad were safer than open field crops by monitoring microbiological, trace metal and pesticide levels via a “farm-to-fork” approach. Open field tomatoes were compared to those grown in greenhouses over a two-year period. Additionally, greenhouse-grown tomatoes and sweet peppers from the university experimental station were analyzed for food safety. Quantitative analyses were based on the presence of 45 pesticide residues, trace metals (lead and cadmium) and microbiological contaminants (faecal coliforms, Staphylococcus aureus and Salmonella sp.). Meals prepared from open field tomatoes in year 2 were analyzed for food safety. Neither greenhouse-grown crops (tomatoes and sweet peppers) nor open field tomatoes had a hazardous quantity of the microbes screened or trace metals, except for a batch of open field tomatoes that contained 0.12 mg/kg lead. Eight pesticides (profenofos, ethion, lambda cyhalothrin, cypermethrin, bifenthrin, iprodione, permethrin, and endosulfan) detected in both the open field and greenhouse-grown crops over a two-year period were present below the EU and Codex MRLs, except for a batch of the university experimental station greenhouse-grown tomatoes and sweet peppers, which exceeded the Codex MRL for lambda cyhalothrin. More pesticides were found in year two due to possible additive effects. Meals prepared using tomatoes posed no health risk to children. Overall, the quality of the open field and greenhouse crops was acceptable for consumers, but annual monitoring of these parameters can assure food safety.
Greenhouse; Open field; Pesticides; Trace metals; MRLs; Tomato; Sweet pepper
Tomatoes (Solanum lycopersicum) and sweet peppers (Capsicum annuum) are nutritious and versatile crops of significant economic importance in the Caribbean. Tomatoes are staples of most farmers in Trinidad and Tobago, but the majority of harvested fruits are produced in East Trinidad, where fruits are sold either wholesale or retail to markets, supermarkets, and food service industries such as hotels and restaurants [1]. Some of these crops are even exported to other Caribbean Community and Common Market (CARICOM) countries to supplement the local demand. Hence, there is a need for crops to be of high quality to ensure food security and the health and wellbeing of consumers. In general, the typical distribution chain of fresh produce can be grouped into three major stages: production, marketing, and consumption. As crops move from farm to table, risks from contamination and spoilage can occur at several points in the food value chain (Figure 1), leading to postharvest losses of fresh produce. Such losses are caused by a variety of factors, such as environmental conditions, mechanical injuries, inadequate storage, unsuitable handling, and transportation, in addition to unhygienic practices [2,3].
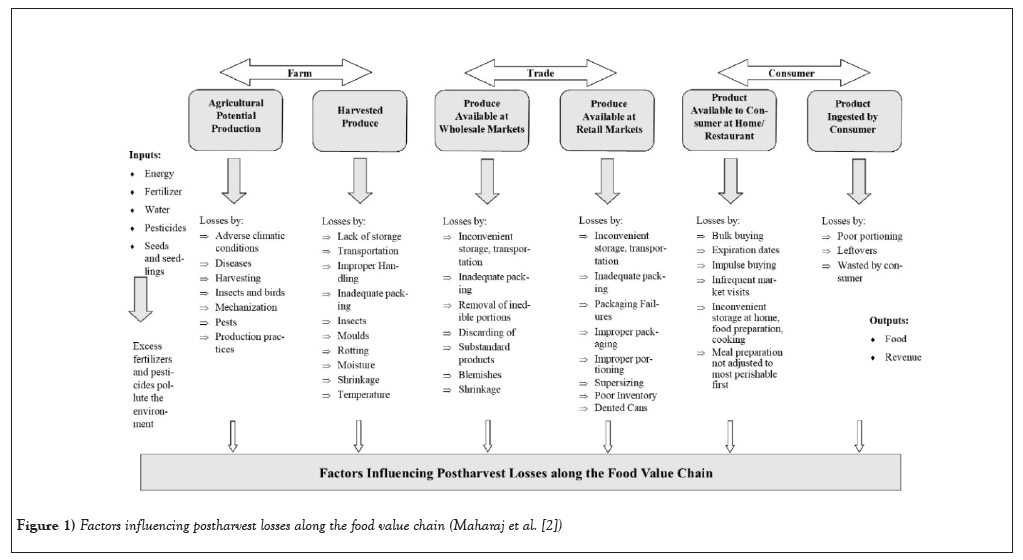
Figure 1: Factors influencing postharvest losses along the food value chain (Maharaj et al. [2]).
Pests and diseases are prevalent in the Caribbean because of the high humidity and temperature conditions. The major pests and diseases associated with tomato production include whiteflies, bugs, beetles, worms, nematodes, viruses, and anthracnose caused by fungi [4]. Plant diseases, which include foliar infections caused by fungi and bacteria that cause major losses in fruit yield, severely hamper sweet pepper production [5]. Over the first half of the fiscal year (2023), sweet pepper production contributed 7.6% to total food production in Trinidad and Tobago, but there was a 40.8% reduction in its production volume compared to that in the same period prior to the year [6]. In many instances, farmers resort to the use of insecticides and pesticides to eliminate plants and crops with visible signs of disease and colonies of insects. Pesticide residues are very small amounts of pesticides or even breakdown products that can remain in or on a crop after harvesting or storage and can make their way into the food chain [7]. The European Union (EU) “farm-to-fork” strategy aims to reduce chemical pesticides by 50% [8]. Protected agricultural structures have several advantages over open field crops because of their temperature and humidity conditions, growth media, pests and diseases, and irrigation, which are better controlled, leading to higher quality crops and yields [9].
Tomatoes were ranked number 12th, and sweet peppers were ranked 7th on the “dirty dozen” list of plants with high pesticide levels [10]. It is important to minimize the exposure of humans, animals, and the environment to these toxic chemicals to prevent long-term effects such as chronic diseases and biomagnification [11]. Countries such as the European Union (EU) have imposed regulations related to the entry of agricultural crops for trace contaminants such as trace metals and pesticides [11]. However, soils for agricultural farming may contain trace metals and nutrient depletion since the parameters of the soil are not being monitored or controlled. There are few studies on trace metal contamination in soils in the Caribbean [12]. Cadmium contamination has been attributed primarily to the preharvest stage, and most mitigation strategies have focused on soil amelioration; however, little research has been conducted at the postharvest stage to identify further points of contamination or on the effects of cadmium contamination on the distribution of trace metals within crops [13]. In greenhouse-grown tomatoes, careful control of potted soil mixes, pesticides, and fertilizers are employed to optimize growth and reduce carbon emissions. Narine et al., [14] reported that 50% of consumers in Trinidad and Tobago were willing to pay more for greenhouse-grown tomatoes. However, the high demand for such tomatoes grown under protected agricultural structures in the Caribbean requires the use of costly chemicals in these smallholder farming systems. Furthermore, consumers and farmers may not be knowledgeable about these toxic chemicals and their levels in apparently healthy-looking fruits, and over time, repeated chemical exposure can have negative consequences due to their systemic effects. Ganpat et al., [15] reported low compliance among farmers with Good Agricultural Practices (GAPs) in Trinidad.
The presence of spoilage microorganisms in crops can be used as a presumptive indicator of the survival of foodborne pathogens. It was estimated that tomato farmers and producers lost an estimated U$250 million following a USFDA recall in 2008 [16] for certain varieties of tomato due to a Salmonellosis outbreak [17]. Salmonella spp. have been reported to cause serious and sometimes fatal infections, particularly in immunocompromised, young, and elderly people.
A vital component of food security is the provision of safe and high-quality foods to consumers. The assessment of food safety practices should be risk- based, evaluating the health risk of consumers from known food safety hazards. Monitoring of farming activities during crop production using appropriate Codex Alimentarius standards of Maximum Residue Limits (MRLs) provides the basis for arriving at credible information for on-farm food safety activities [18]. Laboratory analysis of food samples for food safety hazards provides the means for verifying the presence or absence of food safety hazards that result from food production practices. Thus, monitoring the safety and quality of produce according to national and international standards and regulations is critical not only for determining nutritional and health status but also for marketing products. This study investigated the microbiological and chemical profiles of tomatoes harvested in Trinidad from open fields and greenhouses using sensitive methods over a two- year period. Additionally, greenhouse-grown tomatoes and sweet peppers from the university experimental station were analyzed for food safety. Cooked tomato meals were sampled at school kitchens for microbiological determination of fecal coliforms, Staphylococcus sp. and Salmonella sp. These data can be used to adopt appropriate standards of postharvest practices of food safety and quality for a healthy, market-oriented food supply chain.
Collection of tomatoes and sweet peppers
Tomatoes and sweet peppers were hand-picked by gently twisting the fruit off the stem. Tomatoes from the open field were collected in presterilized bags from a farm in D’Abadie (East Trinidad), as shown on the map in Figure 2, during the dry season on one day in the month of January. Sampling of greenhouse-grown tomatoes was conducted in Wallerfield (East Trinidad). Year 2 samples of tomatoes were harvested from the same open field and the greenhouse used in the first year. Additionally, tomato and sweet pepper harvesting were performed under similar conditions in another greenhouse located at the University of the West Indies Experimental Station, as shown in Figure 3. Sample collection consisted of random selection, and the sampling plan followed the “Recommended Methods of Sampling for the Determination of Pesticide Residues for Compliance with MRLs” [19]. From each growing condition for each crop, the targeted quantity for harvesting and collecting fresh fruits was approximately 30 kg, and approximately 30 samples were separated into 3 groups according to pesticide residue (45 pesticides screened), trace metals (lead and cadmium) and microbiological contamination (faecal coliforms, Staphylococcus aureus and Salmonella sp.). Three independent samples from each crop were used for testing. A further study was carried out to investigate food safety hazards at the cooked product stage, completing from the farm to the table. One-time analyses of uncooked random open field tomato samples from year 2 and cooked school meals were performed for food safety. The meals used were from two school kitchens (A and B) in Trinidad and were of three different types from Kitchen A: Tomato meat sauce/chicken strips (T-Meal 1); spaghetti with tomatoes and vegetables (T-Meal 2); and spaghetti and tomato sauce (T-Meal 3); along with cooked cassava and tomato salads from Kitchen B (CCT Meals 1, 2, 3).
Figure 2: Sample sites in Trinidad.

Figure 3: Greenhouse grown tomatoes and sweet peppers collected in Trinidad and prior to sorting.
Sample preparation
The tomatoes and sweet peppers were sorted for defects and uniformity in size and maturity, with tomato fruits at the mature light-red stage (5-6) and sweet peppers at the mature green stage being selected. The samples were immediately used fresh, washed with deionized water and weighed to ensure that approximately 0.5 kg in triplicate was obtained for each test to ensure sufficient material. After the weights were taken, the entire sample was chopped and homogenized using a Robot-Coupe (USA) high-speed blender. After blending, the sample was filtered using Whatman No. 1 paper, and the sample extracts were stored in amber vials to prevent degradation of the pesticide from light and heat and placed in the refrigerator until processing.
Pesticide analysis using GC-MS
The samples were mixed with sodium sulfate and sodium bicarbonate and extracted with ethyl acetate. The sample extract was then cleaned by solid- phase extraction for GC-electron capture (ECD) analysis. This approach allowed analysis of pyrethrins/pyrethroids and organochlorine pesticides. The extract was cleaned using a carbon-based solid-phase extractor for organophosphorus pesticides via a GC-Flame Photometric Detector (FPD). Reference standards were used for the calibration. Validation was performed using matrix-matched standards. Pesticide residues (organochlorine, organophosphorus and pyrethroids) were tested via Gas Chromatography- Mass Spectrometry (GC-MS). After the data were obtained, they were confirmed with an international standard that showed the Maximum Residual Limit (MRL). The MRL is a value that shows the maximum limit on how much of a pesticide residue can remain on food. For pesticide residue, the filtrate or the supernatant was collected and extracted in an organic solvent (ethyl acetate). The extracts obtained were subjected to GC-MS (Thermo Fisher Scientific, USA) to determine the pesticide concentrations. Analysis of the GC samples was carried out using an Agilent 6890 FPD. Helium was used as the carrier gas at a constant flow rate of 1 mL/min, and the injector temperature was 300°C. A 1 μL sample was injected in split mode. Mass spectra were recorded over a 50-550 m/z mass range with an electron impact ionization energy of 70 eV. The oven temperature increased from 40°C to 320°C. The total running time for each sample was 60 min.
Experiments were performed in duplicate. The chemical components were identified by comparing the retention times of chromatographic peaks using a Quadra pole detector with those of the internal standards. The relative percentage of each component was calculated by comparing its average peak area to the total area. The unit of measurement was mg/kg, and analyses were performed in triplicate.
Trace metal analysis
To 0.25 mL of the sample, was added 4 mL of concentrated nitric acid (HNO3 ) and 1 mL of 30% hydrogen peroxide (H2O2). The microwave digestion of the sample was performed at 190°C for 10 min [20]. The samples were cooled to room temperature and filtered. Analysis of Cadmium (Cd) and Lead (Pb) was performed using a DSQ Thermo Scientific GC-MS (Agilent 6890 GC-FID, ECD) [21]. The same approach was used to prepare standard and blank solutions. Analyses were performed in triplicate, and the results are expressed as mg/kg.
Microbiological analysis
For each batch, 200 g of tomatoes was used for microbiological analyses. Six tenfold dilutions were made from the stock solution. A volume of 100 μL was pipetted onto agar plates in triplicate. Total coliforms (faecal coliforms and E. coli) were enumerated using the pour plate method with Violet Bile Red Agar (VRBA) agar (Oxoid, UK) [22]. S. aureus was enumerated using the spread plate method on Baird-Parker agar (Oxoid, UK) [23]. Pre-enrichment of Salmonella was performed in buffered peptone water, and detection of Salmonella was performed using the spread plate method on Bismuth Sulfite (BS) agar (Oxoid, UK) [24]. The plates were inverted and incubated for 24 h at 37°C. The colonies that were between 20 and 200 were counted and multiplied by the dilution factor to determine the Most Probable Number (MPN) of colonies in the original sample. The results are reported as the average of duplicated samples (Table 1).
| S. No | Organochlorine pesticides and pyrethroids | Agricultural use | Status |
|---|---|---|---|
| 1 | Aldrin | Insecticide | Banned |
| 2 | Allethrin | Insecticide | Approved |
| 3 | Alpha-BHC (Lindane) | Insecticide | Approved |
| 4 | Beta-BHC (Lindane) | Insecticide | Approved |
| 5 | Beta-Cyfluthrin | Insecticide | Approved |
| 6 | Bifenthrin | Insecticide | Approved |
| 7 | Captan | Fungicide | Approved |
| 8 | Chlorothalonil | Fungicide | Approved |
| 9 | Cypermethrin | Insecticide | Approved |
| 10 | Delta-BHC (Lindane) | Insecticide | Approved |
| 11 | Deltamethrin | Insecticide | Approved |
| 12 | Dicofol | Insecticide | Banned |
| 13 | Dieldrin | Insecticide | Banned |
| 14 | Endosulfan I | Insecticide | Approved |
| 15 | Endosulfan II | Insecticide | Approved |
| 16 | Endrin | Insecticide | Banned |
| 17 | Fenpropathrin | Insecticide | Approved |
| 18 | Fenvalerate | Insecticide | Approved |
| 19 | Gamma-BHC | Insecticide | Approved |
| 20 | Iprodione | Fungicide | Approved |
| 21 | Lambda-Cyhalothrin | Insecticide | Approved |
| 22 | Methoxychlor | Insecticide | Banned |
| 23 | o,p-DDE | Insecticide | Banned |
| 24 | o,p-DDT | Insecticide | Banned |
| 25 | Permethrin | Insecticide | Approved |
| 26 | p,p-DDD | Insecticide | Banned |
| 27 | p,p-DDE | Insecticide | Banned |
| 28 | p,p-DDT | Insecticide | Banned |
| 29 | Tolclofos methyl | Fungicide | Approved |
| Organophosphorous pesticides | |||
| 30 | Chlorfenvinphos | Insecticide | Approved |
| 31 | Chlorpyrifos | Insecticide | Approved |
| 32 | Diazinon | Insecticide | Approved |
| 33 | Dichlorvos | Insecticide | Approved |
| 34 | Dimethoate | Insecticide | Approved |
| 35 | Ethion | Insecticide | Approved |
| 36 | Fenitrothion | Insecticide | Approved |
| 37 | Malathion | Insecticide | Approved |
| 38 | Monocrotophos | Insecticide | Banned |
| 39 | Methyl Parathion | Insecticide | Banned |
| 40 | Ethyl Parathion | Insecticide | Banned |
| 41 | Pirimiphos ethyl | Insecticide | Approved |
| 42 | Pirimiphos methyl | Insecticide | Approved |
| 43 | Profenofos | Insecticide | Approved |
| 44 | Prophos | Insecticide | Approved |
| 45 | Triazophos | Insecticide | Approved |
Table 1: Organochlorine and organophosphorus pesticides and their status in Trinidad and Tobago and uses.
Analyses performed at harvest
The 45 pesticides screened were some of the most common pesticides used in Trinidad and Tobago [25] at the time of the study, as indicated in Table 1. There are only three fungicides and 14 out of 42 insecticides are banned in Trinidad (Table 1), although more than 100 pesticides are allowed for use in Trinidad, however the most popular ones were selected. Although 14 of the 45 pesticides are banned for use in Trinidad, it is worthwhile to note whether some of these highly stable organochlorine and organophosphate pesticides persist in the environment (Table 1).
Table 2 shows the detection of 8 of 45 pesticides that were screened from Open Field Tomato (OFT) and Greenhouse Tomato (GT) fruits; and from tomato (UWI-FSGT) and sweet pepper (UWI-FSGSP) fruits from the university experimental station greenhouse at yearly intervals for two years. The pesticide ethion, was detected in tomatoes from open field cultivation in year 1, but no pesticide residues were detected in greenhouse- grown tomatoes during this period; therefore, individual pesticides may not present an unacceptable health risk to the general consumer. Several pesticides were detected in tomatoes from both open field and greenhouse farms and in greenhouse-grown tomatoes and sweet peppers from the university experimental station in year 2. The pesticides included profenofos, lambda cyhalothrin, cypermethrin, bifenthrin, iprodione, permethrin and endosulfan. None of these pesticides are banned in Trinidad and Tobago. Fungicides were not detected, so it was possible that these regimens were abided by more closely than insecticides. The levels of these pesticides did not exceed the Codex or EU MRLs, except for a batch of university experimental station greenhouse-grown tomatoes and sweet peppers, which exceeded the acceptable Codex MRL for the organochloride lambda cyhalothrin and may pose a health risk. Notably, this pesticide was found in year 2 for both crops, confirming the overuse or misuse of lambda cyhalothrin for those crops grown at this location. Therefore, decreasing its usage and retesting crops are recommended to determine whether this pesticide level is safe. More pesticides were found in the year 2 samples, which might be a consequence of more fungal and bacterial infectious diseases and pests posing an increasing threat to crops as resistance has been on the rise. This highlighted the need for having many varieties of these crops to prevent inbreeding and reduce the ability to cope with changing environmental stresses. Endosulfan was found in sweet peppers but not in tomatoes, but it was present at concentrations less than the Codex MRL. No pesticides were detected in open field sweet pepper samples from Central Trinidad locations [26]. This may suggest the need for better greenhouse monitoring of pesticide content. However, there should be some risk assessments as to whether the combined amount of all the pesticide residues in tomatoes and sweet peppers presented an unacceptable health risk.
| Sample code | Pesticide | Level (mg/kg) | Codex MRL (mg/kg) | EU MRL (mg/kg) | Cadmium (mg/kg) | Lead (mg/kg) | Faecal coliforms (MPN/g) | Salmonella sp. (CFU/g) | S. aureus (CFU/g) |
|---|---|---|---|---|---|---|---|---|---|
| Year 1 | |||||||||
| OFT 1 | Ethion | 0.006 | 5 | 0.01 | <0.04 | <0.04 | <3 | n.d. | n.d. |
| OFT 2 | Ethion | 0.005 | 5 | 0.01 | <0.04 | <0.04 | <3 | n.d. | n.d. |
| OFT 3 | Ethion | 0.008 | 5 | 0.01 | <0.04 | <0.04 | <3 | n.d. | n.d. |
| GT 1 | n.d. | N/A | N/A | N/A | <0.04 | <0.04 | <3 | n.d. | n.d. |
| GT 2 | n.d. | N/A | N/A | N/A | <0.04 | <0.04 | <3 | n.d. | n.d. |
| GT 3 | n.d. | N/A | N/A | N/A | <0.04 | <0.04 | <3 | n.d. | n.d. |
| Year 2 | |||||||||
| OFT 1 | Lambda cyhalothrin | 0.007 | 0.05 | 0.1 | <0.04 | <0.1 | <3 | n.d. | n.d. |
| OFT 2 | Lambda cyhalothrin | 0.005 | 0.05 | 0.1 | <0.04 | 0.12 | <3 | n.d. | n.d. |
| OFT 3 | Lambda cyhalothrin | 0.009 | 0.05 | 0.1 | <0.04 | <0.1 | <3 | n.d. | n.d. |
| GT 1 | Cypermethrin | 0.019 | 0.2 | 0.5 | <0.04 | <0.1 | <3 | n.d. | n.d. |
| Bifenthrin | 0.026 | 0.3 | 0.3 | ||||||
| Iprodione | 0.062 | 5 | 5 | ||||||
| GT 2 | Cypermethrin | 0.022 | 0.2 | 0.5 | <0.04 | <0.1 | <3 | n.d. | n.d. |
| Bifenthrin | 0.019 | 0.3 | 0.3 | ||||||
| Iprodione | 0.073 | 5 | 5 | ||||||
| Permethrin | 0.019 | 1 | 0.05 | ||||||
| GT 3 | Cypermethrin | 0.019 | 0.2 | 0.5 | <0.04 | <0.1 | <3 | n.d. | n.d. |
| Bifenthrin | 0.018 | 0.3 | 0.3 | ||||||
| Iprodione | 0.1 | 5 | 5 | ||||||
| Permethrin | 0.025 | 1 | 0.05 | ||||||
| UWI-FSGT 1 | Lambda cyhalothrin | 0.079 | 0.05 | 0.1 | <0.04 | <0.1 | <3 | n.d. | n.d. |
| UWI-FSGT 2 | Lambda cyhalothrin | 0.046 | 0.05 | 0.1 | <0.04 | <0.1 | <3 | n.d. | n.d. |
| <0.04 | <0.1 | ||||||||
| UWI-FSGT 3 | Lambda cyhalothrin | 0.041 | 0.05 | 0.1 | <0.04 | <0.1 | <3 | n.d. | n.d. |
| Year 2 | |||||||||
| UWI-FSGSP 1 | Lambda cyhalothrin | 0.022 | 0.05 | 0.1 | <0.04 | <0.1 | <3 | n.d. | n.d. |
| UWI-FSGSP 2 | Lambda cyhalothrin | 0.012 | 0.05 | 0.1 | <0.04 | <0.1 | <3 | n.d. | n.d. |
| Endosulfan | 0.04 | 5 | 0.05 | <0.04 | <0.1 | <3 | n.d. | n.d. | |
| UWI-FSGSP 3 | Lambda cyhalothrin | 0.058 | 0.05 | 0.1 | <0.04 | <0.1 | <3 | n.d. | n.d. |
| Uncooked tomato from open field in Year 2 used in meal preparation for school children | |||||||||
| Uncooked T 1 | Profenofos | 0.067 | 0.05 | 10 | < 0.04 | <0.04 | <3 | n.d. | n.d. |
| Uncooked T 2 | Profenofos | 0.13 | 0.05 | 10 | < 0.04 | <0.04 | <3 | n.d. | n.d. |
| Uncooked T 3 | None | N/A | N/A | N/A | < 0.04 | < 0.04 | <3 | n.d. | n.d. |
| T-Meal 1 | N/A | N/A | N/A | N/A | < 0.04 | <0.04 | <3 | n.d. | n.d. |
| T-Meal 2 | N/A | N/A | N/A | N/A | < 0.04 | <0.04 | <3 | n.d. | n.d. |
| T-Meal 3 | N/A | N/A | N/A | N/A | < 0.04 | < 0.04 | <3 | n.d. | n.d. |
| CCT-Meal 1 | N/A | N/A | N/A | N/A | < 0.04 | <0.04 | <3 | n.d. | n.d. |
| CCT-Meal 2 | N/A | N/A | N/A | N/A | < 0.04 | <0.04 | <3 | n.d. | n.d. |
| CCT-Meal 3 | N/A | N/A | N/A | N/A | < 0.04 | < 0.04 | <3 | n.d. | n.d. |
Note: n.d-not detected; OFT-Open Field Tomato; GT- Greenhouse Tomato; UWI-FSGT-UWI Field Station Greenhouse Tomato; UWI-FSGSP-UWI Field Station Greenhouse Sweet Pepper; T-Cooked Tomato; CCT-Cooked Cassava and Tomato Salad; S. aureus-Staphylococcus aureus.
Table 2: Food safety analyses of open field and greenhouse harvested crops from Trinidad before and after processing.
Cadmium and lead have been previously found in several soils due to leaching from nearby water sources. These compounds have neurotoxic and mutagenic effects, so it is important to assess their content in crops. Greenhouse soils are usually controlled, for example, by the use of potting mixes; thus, trace metal contamination does not pose a great risk. Table 2 shows the cadmium and lead contents of tomatoes harvested from open fields and from greenhouses. The concentrations of the trace metals lead and cadmium in the open field or greenhouse-grown tomatoes from year 1 were less than those in the Codex MRLs and therefore did not pose an unacceptable health risk. One of three samples of open field tomatoes in year 2 had lead contents that exceeded the Codex MRL and therefore may present an unacceptable health risk. The cadmium levels in the samples of open field tomatoes and the lead and cadmium levels in greenhouse-grown tomatoes were below the Codex MRLs and therefore were considered safe. There was a negligible content of trace metals in the greenhouse–grown sweet peppers (Table 2). Neither the greenhouse–grown tomatoes nor the sweet peppers tested positive for cadmium or lead, suggesting that the controlled soil preparations or potting mixes were more suitable for these crops than were soils in open fields.
As a general observation, it can be inferred from the data in Table 2 that, using the guidelines of the relevant Codex standards as the reference, overall, the food safety practices of farmers during crop production, including preharvest, harvest and postharvest transportation activities, were acceptable, and there appeared to be no unacceptable health risk from biological food safety hazards or unsanitary practices. Farmers can benefit from formal training on the safe use of agricultural chemicals for crop production and, in some instances, do self-training. Farm checks on equipment used for spray application of agricultural chemicals are generally not calibrated; therefore, the ability of the equipment to deliver the recommended application of chemicals is questionable. Farmers may not keep written records of the agricultural chemicals that they apply to crops, and they do not have access to water quality information for the water that they use in food production systems (Table 2).
Postharvest practices
Few studies exist that follow the safety parameters of fresh produce via a “farm-to-fork" approach. Table 2 also shows the safety parameters of the prepared school meals. This study can serve as a model for the food industry to follow for safety and quality assurance as most food outlets in Trinidad and Tobago do not utilize organic produce for meal preparation since market-bought produce is usually cheaper.
Tomatoes from a random farmer’s open field in year 2 were purchased from the local market in Trinidad and were also tested for the presence of pesticides, as illustrated in Table 2. These tomatoes were used to prepare meals in the school kitchen. Two of the samples contained profenofos pesticide residues that were below the EU MRLs and therefore may not present an unacceptable health risk to the general consumer. These results point to the use of crops treated with pesticides in the preparation of meals for children. However, further monitoring is recommended to determine whether pesticides are misused. The market tomatoes used in this study were found to have profenofos, which was not found in either the open field or greenhouse test samples. Hence, monitoring studies must be performed across a larger number of production sites and markets to determine pesticide traceability from farms to consumers in the distribution chain.
Three tomato-based samples of cooked or prepared meals from an approved school kitchen, along with a fresh uncooked tomato sample from a batch of open field year 2 tomatoes, showed no trace metals or microbial contamination (Table 2). Similarly, another school kitchen, which included a hot cassava salad, was used to prepare school meals using similar open field tomatoes and cassava (Table 2). Only microbial and trace metal analyses were carried out and no pesticides were analysed in these samples due to interference of the fats in the prepared meals with the pesticide components, which would have masked the results. Thus, only fresh uncooked tomatoes were used for pesticide analysis. Microbiology analyses were performed on meals from both school kitchens A and B (Table 2) to assess the cleanliness and safety of the cooking practices employed. There was no unacceptable food safety health risk associated with pathogenic/or indicator microorganisms (biological food safety hazards) associated with tomatoes, or other crops used in prepared meals in school kitchens. Simple washing or peeling can remove any pesticide or microbe. Microbes are also killed during heating.
However, risk assessments should be performed to determine whether the total amount of pesticide residue (combined) in tomatoes presents an unacceptable health risk to students in school meal programs. The outer parts of tomatoes and sweet peppers are commonly used in cooking so not detecting these hazards in cooked products was an indicator of good food handling practices in these two kitchens. This approach is important because kitchens serve thousands of students daily on weekdays. However, follow-up services should be offered to assure quality.
A study on pesticide use in produce from “farm-to-fork” in Uganda was performed, and organophosphates (91.3%) and pyrethroids (60.0%) were found to be prevalent, similar to the findings of the present study [27]. While pesticides decreased as they moved up the value chain in this study, Ssemugabo et al., [27] found both decreases and increases. A greater variety of foods can be sampled to make more definitive conclusions in this study. There are few monitoring studies on pesticides that have been performed in Trinidad and Tobago and the Caribbean with fresh produce, and even fewer studies have been conducted on trace metal levels. It is possible that the products exported from Trinidad and Tobago may have violated the Codex MRLs, which has implications for food safety because they have not been continually monitored. In Trinidad and Tobago, no MRLs for pesticides or related standards for pesticide residues on agricultural produce have been published for farmers as a reference or guide. In 1999, a market basket survey showed that 10% of produce exceeded Codex MRLs [28]. A study performed in Trinidad found that tomatoes collected from the Chaguanas market, Central Trinidad, contained the organophosphate diazinon, at 0.50 ± 0.03 mg/kg, which is above the EU MRL (0.01 mg/kg) but within the range for the Codex MRL (0.5 mg/kg) and was considered safe for consumption [26]. This pesticide was not identified in this study. In the wider Caribbean, several studies have been conducted on pesticides in tomatoes. In Suriname, tomatoes from the market were found to contain the pyrethroid cypermethrin at an MRL of 0.32 ± 0.02 mg/kg, which was above the US and WHO recommended MRL (0.2 mg/kg) but within the range of the EU MRL (0.5 mg/kg) and was considered safe [29]. In these studies, similar broad panels were used for GC-MS analyses, but this approach is expensive and time consuming. Despite Jamaica having set MRLs, unmonitored pesticide levels in produce are a cause for concern [30]. It was projected that 8.8% of produce cannot be exported to other countries if the produce is not below the MRLs [31]. Hence, a lack of testing decreases export capacity.
A study on the trace metal content of 21 brands of rice in Trinidad found the absence of lead and other trace metals below the Codex MRLs [32]. The arsenic (As), aluminum (Al), lead (Pb) and cadmium (Cd) contents of fresh produce such as tomatoes, sweet peppers, cassava, and carrots were investigated in Jamaica [33]. The Pb content (0.021 mg/kg) and Arsenic (As) content (0.012 mg/kg) were within the Codex MRLs, but the Al content (12.89 mg/kg) and Cd content (0.266 mg/kg) exceeded the Codex MRLs [33].
Sometimes plants are not exposed to the same pathogens or inhibitors due to differences in the environment. Consequently, certain genes expressing particular gene products may not be up-regulated. This is a common challenge for plants harvested from farms versus greenhouse-grown plants, where the latter may be more regulated. The twin island of Trinidad and Tobago has more than 100 approved pesticides. Farmers may use unregulated amounts of pesticides in combination with pesticides that are not effective against pathogens or pests that attack a particular crop. This nonspecific approach leads to unnecessary toxins being present in the crop. Education is important for reducing these occurrences to improve the quality of tomatoes grown locally. Research has been conducted in Trinidad using seaweed extracts as bio pesticides which are attractive alternatives for mitigating this problem [34]. There is an increasing demand for organically grown tomatoes, which can reduce the health risks posed by pesticides.
Several soils across various locations in East Trinidad contain trace metals [35]. Trace metal uptake in plants may occur inadvertently across ion channels meant for useful ions and thus be found in fruits. Unlike pesticides, which can be removed by washing or peeling, trace amounts of these metals can be found in the flesh of fruits. The potential threat of either pesticide or trace metal contamination must not be treated lightly if food security is to be enforced in the Caribbean. The study suggested no significant difference between the contaminant trends for two years for greenhouse and open field tomatoes but further testing is required to validate these claims.
The study had several limitations. The full gamut of pesticides in Trinidad were not screened. Secondly, the rate of applied pesticides dissipation in the examined fields and greenhouses could not be determined. More replicates would have been required for each year for both the crops and the meals to validate the trends observed or make any correlation. Interviews also need to be conducted to determine the source of the produce to track it from farm to fork. The pesticides used currently would be very different to those used prior so the study needs to be repeated to make conclusions that can be applicable currently. It might also be worthwhile to determine the quantity of micro plastics in tomatoes since they have been accumulating in soils in addition to heavy metals. Another recommendation is to test the soil in which the plants are growing to determine the percentage of heavy metals or pesticides that are migrating into the plant.
Crops in the production, marketing and distribution chains were evaluated for food safety hazards in terms of pesticide residues, cadmium and lead trace metals and microbiological hazards. Ethion pesticide residue, which is approved on the Codex list of pesticides, was detected in open field tomatoes in Trinidad. While it is an approved pesticide in Trinidad and the levels are within the EU MRLs, further monitoring is recommended to determine whether this pesticide could be misused. With respect to the greenhouse– grown tomatoes, no pesticides were detected in year 1 samples; in contrast, for tomatoes grown in the open field, the levels of cadmium and lead were less than the Codex MRL values of 0.05 and 0.1 mg/kg, respectively. Additionally, no microbial contamination was detected. Pesticides were also detected in year 2 samples of open field and greenhouse-grown tomatoes, but these were within the Codex MRLs. However, greenhouse tomato samples from the university experimental station had average pesticide levels for lambda cyhalothrin above the Codex MRLs compared to those of the university experimental station greenhouse-grown tomato samples. Based on these results, from both monitoring crop production practices and limited laboratory analysis of tomatoes and sweet peppers, it would be worthwhile to extend these studies for a longer study period and perform these studies on other economically important crops. As such, baseline data can be built upon for other economically important crops and at other time points, and improvements in the quality of produce in the Caribbean can be monitored to guide farming practices.
Along with education and outreach activities at the farm level, food safety practices and the use of portable equipment to test for the presence of food safety hazards in the field are recommended for ease of monitoring, efficiency and frequency in the Caribbean.
The authors wish to acknowledge the support of McGill University, The Caribbean Industrial Research Institute (CARIRI) and The University of the West Indies, St. Augustine Campus.
Rohanie Maharaj and Inteaz Alli contributed to the study conception and design. Rohanie Maharaj performed the field work and both she and Farrah Mathura completed an initial draft of the paper. Furthermore, Farrah Mathura and Rohanie Maharaj made significant edits to the paper, formatted the tables and updated the literature and references. All the authors commented on previous versions of the manuscript and read and approved the final manuscript.
This study was a component of a joint research collaboration between McGill University and The University of the West Indies, St. Augustine Campus. We acknowledge the support and partial contributions of The University of the West Indies under the CRP grant CRP.3.NOV21.01-“Rapid Testing of physical, chemical, and microbiological contaminants in the Food Science and Technology Laboratories at the University of the West Indies, St. Augustine.”
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Maharaj R, Mathura F, Alli I. Pesticide, trace metal and microbiological analyses of open field and greenhouse-harvested crops from Trinidad before and after processing. AGBIR.2024;40(2): 926-932.
Received: 01-Jan-2024, Manuscript No. AGBIR-24-125644; , Pre QC No. AGBIR-24-125644(PQ); Editor assigned: 03-Jan-2024, Pre QC No. AGBIR-24-125644(PQ); Reviewed: 17-Jan-2024, QC No. AGBIR-24-125644; Revised: 26-Jan-2024, Manuscript No. AGBIR-24-125644(R); Published: 05-Feb-2024, DOI: 10.35248/0970-1907.24.40.926-932
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
