Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2024) Volume 40, Issue 3
An enzyme with several industrial and commercial uses is pectinase. The general use of pectinase may be severely limited, nevertheless by greater production costs. Hence, employing a distinct substrate and refining the components of the fermentation medium and process conditions, researchers are working to lower the cost of pectinase production for eventual application in industrial operations. The major goal of the current work was to improve the medium composition for Aspergillus niger-based solid-state fermentation to produce pectinase. Citrus pulp’s pectin can be hydrolyzed by pectinases in citrus waste bio refineries to create galacturonic acid, a potential platform chemical. The pectinase enzyme was produced by Aspergillus niger grown on orange peel as a sole carbon source under solid state and liquid fermentation. The purification process begins with the concentration of crude enzyme using Ammonium sulfate precipitation. The optimal temperature and pH were 50°C and 8 respectively. The maximum production of pectinase in the presence of cheaper substrate at very low concentration makes the enzyme useful in industrial sector for juice and jams, jelly and textile.
Fermentation; Aspergillus niger; Pectinase; Galacturonic acid; Solid state fermentation
Oranges, scientifically known as Citrus sinensis, are universally recognized as a crucial and immensely popular tropical fruit, often occupying the top spot on the list of super fruits. Belonging to the Citrus genus within the Rutaceae family, oranges have secured remarkable production and consumer acceptance.
Enzymes, remarkable molecules present in minute quantities within the cells of living organisms, possess the extraordinary ability to accelerate chemical reactions without undergoing any change themselves. The advantages offered by enzymes over traditional chemical catalysts are manifold, including their exceptional specificity, high catalytic efficiency and adjustable activity [1,2]. These attributes have elevated the utilization of enzymes across diverse industries, spanning pharmaceuticals, chemicals and food production [3]. Consequently, the demand for industrial enzymes has witnessed a substantial surge, prompting ongoing research and development efforts aimed at optimizing output while conserving resources [3].
The exploration of enzymes dates back to the mid-nineteenth century, marked by the industrial application of fungal enzymes as pioneers in this field [4]. Since then, enzyme research has evolved significantly, propelled by the recognition of their pivotal role in various industrial processes.
Pectinases serve the essential function of breaking down pectin compounds characterized by methyl esterified and 1,4-glycosidic-bonded structures [5]. These heterogeneous pectin substances primarily consist of pectins, protopectins and pectinic acids, also referred to as polygalacturonic acids [6]. The categorization of pectinases is based on their specific modes of action, resulting in four distinct types: pectin esterases, pectin lyases, polymethylgalacturonases and pectate lyases. These enzymes facilitate-elimination reactions, leading to the formation of galacturonides. Noteworthy among pectinases are polygalacturonases, pectin lyases and pectate lyases which demonstrate the highest enzymatic activity. Interestingly, these pectinolytic enzymes exhibit sequence-level similarities, highlighting their common ancestry.
Pectinases are predominantly sourced from bacteria, fungi and plants, with notable contributions from Bacillus, Aspergillus, Saccharomyces species, among others [7]. The production of pectinases is achieved through submerged and solid-state fermentations, enabling the generation of substantial enzyme quantities. The wide-ranging applications of pectinases encompass critical roles in the food industry, oil extraction, textile manufacturing and the fermentation of coffee and tea. Particularly, acidic pectinases play a pivotal role in the juice clarification process, notably enhancing juice yields through the liquefaction of pulp [8].
Pectinases represent a diverse category of enzymes primarily found in higher plants and microorganisms, specializing in the hydrolysis of pectic compounds. The term “pectinases” encompasses a collective of enzymes that collectively break down these pectic compounds. Notably, pectinase includes the enzymatic components Polygalacturonase (PG), Pectin Lyase (PL) and Pectinesterase (PE) all of which act on pectin compounds [9,10].
In the process of pectin degradation, pectinase is capable of breaking down intricate pectin polysaccharides into smaller units, predominantly galacturonic acid molecules. Among these enzymes, Polygalacturonase (PG) stands out as one of the most widely utilized pectinases [11]. Its initial characterization revealed that PG functions as a pectinase, effectively catalyzing the hydrolysis of polygalacturonic acid within an aqueous environment.
Another vital member of the pectinase family is pectin lyase, which operates by inducing a β-elimination racemate. This mechanism leads to the cleavage of glycosidic bonds at the C-4 position while simultaneously removing hydrogen atoms from the C-5 position. This complex process results in the creation of an unsaturated product containing a double bond [12].
Pectinesterase (PE) is responsible for the hydrolysis of methyl ester linkages in the galacturonic acid chain of pectin. This action produces pectin acid and methanol as byproducts [13].
In summary, pectinases constitute a diverse group of enzymes present in higher plants and microorganisms and they play a crucial role in breaking down pectic compounds. The pectinase category encompasses enzymes such as polygalacturonase, pectin lyase and pectinesterase, each contributing to the degradation of pectin substances in unique ways.
Pectinase emerges as a potent biocatalyst with the remarkable capability to convert pectin into galacturonic acid [14]. This enzymatic marvel constitutes a significant portion, approximately 10%, of the globally produced industrial enzymes and its presence in the market continues to experience steady expansion. A diverse range of microorganisms partake in the production of pectinase, including bacteria, fungi, Actinomycetes, Pseudomonas, Bacillus, Saccharomyces and Aspergillus [15-21].
Of noteworthy mention is the security-approved Aspergillus strain, classified as “Generally Recognized As Safe” (GRAS) by the United States Food and Drug Administration (USFDA). The metabolites derived from this strain find versatile application across numerous industries, encompassing feed production, biotechnology, paper manufacturing, textiles, pharmaceuticals and environmental sectors [22]. The utilization of Aspergillus species and their metabolites within the agricultural sphere, particularly for fruits, vegetables and various crops, has consistently yielded favorable economic outcomes [23].
In summary, pectinase stands as a formidable biocatalyst capable of transforming pectin into galacturonic acid. This enzyme occupies a substantial proportion of global industrial enzyme production, with its prevalence in the market continually expanding. The collaborative efforts of diverse microorganisms, including Aspergillus, contribute to the generation of pectinase. Aspergillus, recognized as GRAS by the FDA, holds the potential for versatile application in multiple industries, while its use in agriculture has demonstrated positive economic benefits.
Fungi, recognized for their ability to produce pectinase with robust enzymatic efficacy at a reasonable cost, have been somewhat understudied in this domain. Nevertheless, their widespread application spans numerous industrial domains, including animal feed production, pulp and paper manufacturing, pharmaceuticals, fermentation processes, juice and oil extraction, coating procedures and wastewater treatment [24,25]. Explorations into citrus peel variations have unveiled substantial pectin content within certain types of citrus peels. Notably, citrus stands out as a commercially viable and popular crop, celebrated for its nutritional advantages and secondary metabolites [26,27]. Given these attributes, citrus peel emerges as a pivotal substrate for the production of a variety of enzymes, pectinase included [28,29].
Around 10% of the global enzyme market is composed of pectinolytic enzymes, harnessed across diverse industries, particularly in the clarification phase of wine and fruit juice production. The current trend centers on performing these processes at lower temperatures to curb the growth of unwanted bacteria, preserve labile and volatile flavor components and conserve energy. This pursuit has led to the ongoing quest for cold-active pectinases, as the presently employed pectinases display limited effectiveness below 35°C, while being highly efficient at around 50°C. Preliminary research has unveiled promising pectinolytic activity in yeasts and yeast-like microorganisms isolated from Antarctica.
Collection of orange fruit waste and substrate preparation
Orange peels were procured from a market stall located in Waghodia, Vadodara, Gujarat. These peels underwent a preparatory process that involved cutting them into uniformly sized small pieces. Subsequently, the pieces were spread out on trays and subjected to natural sunlight exposure until they reached a consistent weight. Once this weight was achieved, the dried peels were finely ground into a powdered form using an electric grinder. This powdered orange peel material was then utilized for Solid-State Fermentation (SSF) as part of the subsequent processes (Figure 1).

Figure 1: Orange peels powder.
Isolation of Aspergillus niger species
We used Potato Dextrose Agar (PDA) media for the growth of Aspergillus niger species. Composition of PDA potato infusion 200 gm, dextrose 20 gm, agar 20 gm, distilled water 1 litre. Media was autoclaved at 121°C for 20 min. After media will autoclaved Aspergillus niger was inoculated by spread plate method (Figures 2 and 3).

Figure 2: Isolation of Aspergillus niger species.

Figure 3: Microscopy study of Aspergillus niger.
Primary screening for pectinase production
Pectin broth preparation: To check the activity of pectinase pectin broth was prepared composition of pectin broth 1 g pectin, 1 g yeast extract, 0.5 g Sodium Chloride (NaCl) in 100 ml distilled water. We are prepared 2 pectin broth medias one for inoculation and other for control. Take 2 conical flask of 250 ml add pectin broth media in both conical flask was autoclaved at 121°C for 20 min. After media will cool down add one disc of actively growing Aspergillus niger in media. After 5-6 day’s mycelium will appear (Figure 4).

Figure 4: Degradation of pectin substance.
Polygalacturonase assay: Pectinase activity was carried out using 3,5-Dinitrosalicylic Acid (DNSA) method. By using phosphate buffer (pH 7) and 1% Pectin.
Prepare 1% Pectin preparation, add 1 gm pectin in 100 ml distilled water, dissolve by heating in microwave. Take 1 ml of 1% pectin in 3 test tube and add 0.5 ml of inoculated broth and 1 ml DNSA and add 1 ml phosphate buffer (pH 7) and prepare 1 blank as 1 ml distilled water and 0.5 ml of inoculated broth and 1 ml DNSA and add phosphate buffer (pH 7) Keep these test tubes in boiling water bath (above 100 temp) for 10 minutes cool the test tubes and add 9.5 ml distilled water in test tube and mix properly and take Optical Density (OD) at 540 nm in spectrophotometer.
Pectin hydrolysis: Pectin hydrolysis can see by iodine test. The clear zone is visible. Pectinase enzyme hydrolysed the pectin, which is clearly visible in plate (Figure 5).
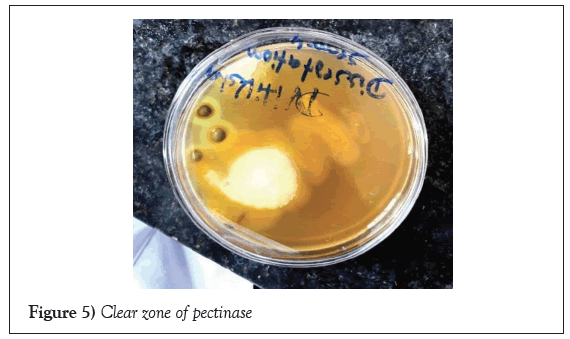
Figure 5: Clear zone of pectinase.
Fermentation
Aspergillus niger producing pectinase enzyme. This pectinase enzyme degrading pectin substance (Orange peel powder) which is present in media.
Liquid state fermentation: Take two 250 ml Erlenmeyer flasks containing 100 ml of sterile cultivation medium made up of 0.1% ammonium nitrate (NH4NO3), 0.1% magnesium sulfate heptahydrate (MgSO4.7H2O), 0.1% ammonium dihydrogen phosphate (NH4H2PO4) and 1% ground orange peel was autoclaved at 121°C for 20 min. Then add a disc of Aspergillus niger in 1 flask and one will act as a control (Figure 6).

Figure 6: Liquid fermentation of pectinase.
Solid state fermentation: Take two 250 ml Erlenmeyer flasks containing 15 ml of sterile cultivation medium made up of 0.1% NH4NO3, 0.1% MgSO4.7H2O, 0.1% NH4H2PO4 and 15 g ground orange peel was autoclaved at 121°C for 20 min. Then add a piece of Aspergillus niger in a flask and one act as a control (Figure 7).

Figure 7: Solid fermentation of pectinase.
Filtration
Fermented media will filtrate with the help of filter paper then extracted media will centrifuge for 15 min at 4000 RPM (Figure 8).

Figure 8: Extraction of pectinase by liquid and solid fermentation.
Characterisation of pectinase
Effect of pH on enzyme: The effect of reaction pH on pectinase activity was accessed using phosphate buffer (6, 6.5, 7, 7.5, 8, 9) acetate buffer (pH 3, 4). The reaction mixture was incubated for 30 min at 37°C. The pH stability of the purified pectinase was determined by pre-incubation of the enzyme at various pH range (pH 3.0-9.0) for 20 min. The residual activity was measured by the standard method.
Most enzyme have characteristic pH at which their activity is maximal, above or below this activity declines. The enzymes and substrate together to form a complex. This binding of the enzyme takes place on a specific site on the enzyme called as active site and the nature of binding is mainly electromagnetic depending on the charge available on the substrate and the active site. Since it is composed of amino acid change varying with p H it follows that substrate that binding to an enzyme will be affected by pH of the medium. Pectinase activity is highest at pH 8.
Effect of temperature on enzyme: The effect of incubation temperature (5°C, 10°C, 20°C, 30°C, 37°C, 40°C, 45°C, 50°C, 55°C, 60°C) on pectinase activity was determined by incubation of the reaction mixture in 37°C, Phosphate buffer (pH 8) for 30 min at each temperature.
The optimum temperature is the result of the balance between the rate of increase of activity and rate of denaturation of enzyme. The optimum temperature is not a constant value for a given enzyme but depends upon the time during which the measurement of activity are made. Shorter the time of measurement, higher will be the apparent optimum temperature. The Enzyme activity is highest at temperature 50°C.
Effect of substrate concentration of enzyme: Effect of substrate concentration on pectinase activity was determined by the enzyme with 0.2, 0.4, 0.6, 0.8, 1.0 ml and 0.5 ml of orange pectin. We are using phosphate buffer at 50°C.
The effect of substrate concentration on the enzyme activity have lot of application which help in understanding the characteristics of enzyme have been determine. The enzyme activity for various substrate concentration is determine. The graph is increased as the concentration of substrate increases.
Effect of enzyme concentration: Effect of enzyme concentration on pectinase activity was determine by orange enzyme with 0.1, 0.2, 0.3, 0.4, 0.5 ml and 1 ml of pectin. We are using phosphate buffer with pH 8 at 50°C.
The effect of enzyme concentration on the enzyme activity have lot of application which help in understanding the characteristics of enzyme have been determine. The enzyme activity for various enzyme concentration is determine. The graph is increased as the concentration of substrate increases and at the end it drawn as the enzyme concentration.
Purification of pectinase
Ammonium sulphate purification: The supernatant was precipitated with 80% saturation of solid ammonium sulphate at 4°C. Precipitation was allowed to continue overnight, which was followed by centrifugation at 4000 RPM for 15 min. Supernatant was collected and activity was checked (Figure 9).

Figure 9: Purified enzyme from liquid fermentation and solid fermentation.
Clarification of fruit juices
Apple, orange, pineapple juice collected from a stall at Limda, Vadodara (Figure 10).

Figure 10: Clarification of apple, orange, pineapple juices.
Biuret test
Biuret test is used to check the presence of reducing sugar in sample. If the purple colour is present, it indicates the presence of protein (Figure 11).
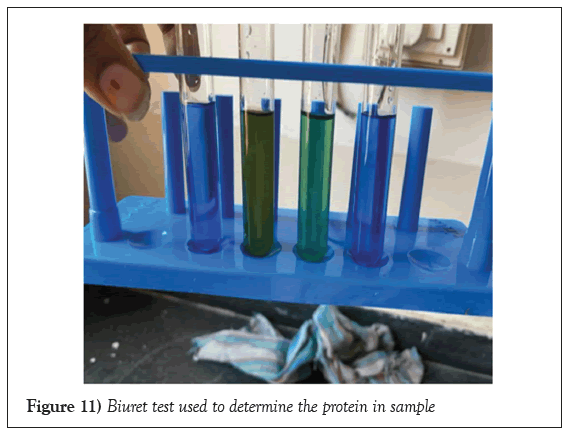
Figure 11: Biuret test used to determine the protein in sample.
Bradford’s test
Bradford’s test is used to check the presence of protein in sample, add 2-3 drops of purified and unpurified sample in tubes and add 2-3 drops of reagent (Figure 12).

Figure 12: Bradford reagent used to identify the presence of protein.
The isolation of pectinase from Aspergillus niger obtained from orange peels proved to be highly effective. The biochemical characteristics of this pectinase enzyme indicate its potential suitability for various biochemical reactions in industrial biotechnological processes, particularly within the juice and food industries.
Microbial pectinases dominate the industrial sector, finding extensive application across various industries with ongoing development of new applications. However, to make these processes cost-effective, considerations of enzyme titre and stability become crucial. While several researchers have reported pectinase production using cost-efficient substrates, challenges remain, often stemming from poor enzyme activity or instability at high temperatures over prolonged periods. Consequently, maintaining enzymes at lower temperatures contributes to increased costs for industrial utilization. Pectinase-mediated juice clarification plays a vital role in extending the shelf life of commercially produced and transported juices, reducing the risk of microbial contamination.
The filamentous fungus Aspergillus niger is known for its versatile growth capabilities on a wide range of surfaces and its presence is widespread across the globe. While some species of Aspergillus niger find applications in various industries, including fermentation for the production of hydrolytic enzymes like amylases and lipases, as well as organic acids such as citric and gluconic acids, certain strains can also contribute to food spoilage [30]. Furthermore, filamentous fungi like those from the Aspergillus genus, such as Aspergillus niger, have garnered significant use in biotechnological processes as cellular factories. This is attributed to their metabolic adaptability and capacity to secrete substantial quantities of antibiotics, polysaccharides, enzymes, vitamins and their suitability for applications such as treating textile wastewater and dyes [31].
The specific fungal strain employed in the present study was previously isolated from orange peels. Orange peels provide an environment conducive to Aspergillus niger growth due to the presence of soluble sugars and pectin, which serve as primary components. Orange peels comprise various constituents, including fiber (pectins: 42.5%, hemicelluloses: 10.5%, cellulose: 9.21%, lignin: 0.84%), soluble sugars (16.9%), protein (6.5%), starch (3.75%), ashes (3.5%) and fats (1.95%) [32]. Notably, the total sugar content in orange peels can vary within a range of 29% to 44% [33].
The present study builds upon prior research by modifying fermentation parameters for enhanced pectinase production, involving variables such as pH, temperature and substrate concentration. The investigation underscores the potential of naturally occurring soil microflora in degrading pectin waste. Further research can focus on studying pectinase enzymes from diverse microorganisms to identify efficient pectinase producers and analyze turnover rates. Zymogram analysis offers insights into the molecular weight and activity of the pectinase enzyme.
Two distinct protein estimation methods, namely the Bradford and biuret methods, were employed to detect protein, in this case, the pectinase enzyme. The Bradford method qualitatively confirms protein presence, while the biuret method allows for quantitative estimation. Results indicate that the partially purified enzyme exhibits twice the activity of the crude sample, along with a relatively lower protein content.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Rohinkar A, Jadav N, Sailaja I. Partial purification and characterisation of pectinase produced by Aspergillus niger grown on orange peels as a substrate. AGBIR.2024;40(3):1037-1041.
Received: 11-Mar-2024, Manuscript No. AGBIR-24-127721; , Pre QC No. AGBIR-24-127721 (PQ); Editor assigned: 14-Mar-2024, Pre QC No. AGBIR-24-127721 (PQ); Reviewed: 29-Mar-2024, QC No. AGBIR-24-127721; Revised: 05-Apr-2024, Manuscript No. AGBIR-24-127721 (R); Published: 12-Apr-2024, DOI: 10.35248/0970-1907.24.40.1037-1041
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
