Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2024) Volume 40, Issue 5
Chickpea is an annual legume crop grown in temperate and subtropical regions. It belongs to the Fabaceae family and is native to Southwest Asia. Chickpea have been cultivated since ancient times in both Asia and Europe. India stands as the largest producer and consumer of chickpea globally, contributing roughly 75% of the world's total production. It is primarily consumed in various processed forms, such as boiled, roasted, fried or steamed whole seeds as well as in the form of dal and dal flour. They are commonly used in the preparation of snacks, sweets and condiments. Chickpea crop is prone to various diseases like, dry root rot (Rhizoctonia bataticola), Fusarium wilt (Fusarium oxysporum f. sp. ciceri), wet root rot (Rhizoctonia solani), collar rot (Sclerotium rolfsii), Aschochyta blight and Sclerotinia rot (Sclerotinia sclerotiorum (Lib.) de Bary). Among various diseases, Sclerotinia rot, also known as stem rot, caused by Sclerotinia sclerotiorum (Lib.) de Bary, is one of the most significant. It causes substantial yield losses, especially in cool and moist areas conducive to its development. The fungus's explosive pathogenicity and the resilience of its sclerotia contribute to its success as a pathogen. Due to its strict soil-borne nature and wide host range, managing this disease through host resistance or chemical management alone is challenging. Fungicides are commonly used to control plant diseases, but they pose environmental and health risks and can lead to environmental hazards. Therefore, integrated disease management using both bio-agents and fungicides is the best alternative. The results showed that Sclerotinia rot incidence in chickpeas was significantly reduced and yield increased when chemical and biocontrol agents were used together, compared to using either chemicals alone or biocontrol agents alone. Among the different treatments, the highest percent of disease control (81.40%) was found in T12-Seed Treatment (ST) with tebuconazole 2% DS at 1.5 g/kg seed+Soil Application (SA) at 10 kg/ha with Trichoderma harzianum, followed by T11-ST with tebuconazole 2% DS at 1.5 g/kg seed+SA at 10 kg/ha with Trichoderma viride (80.33%). The highest disease incidence was found in T8-ST with thiophanate methyl 70% WP at 2 g/kg seed (37.77%).
Biocontrol agents; Chickpea; Fungicides; Integrated disease management; Sclerotinia rot
Cicer arietinum, commonly known as chickpea, is an annual crop that grows in temperate and subtropical zones and is native to Southwest Asia. It is also called by various names such as gram, Bengal gram, garbanzo, garbanzo bean, Egyptian pea, chana and chole in various places. Pulses are the important source of protein in the diets of the poor and are particularly important in vegetarian diets. It is cultivated from ancient times both in Asia and European countries. India is the largest producer and consumer of chana in the world. India accounts approximately 75% of world’s chickpea production. Mainly there are two types of varieties of gram desi and kabuli. Approximately 80% of the chana produced in world is of Desi type and the remaining is of kabuli variety. In India, Desi variety is also cultivated massively.
It is mainly consumed in the form of processed complete seed (boiled, roasted, fried, steamed, etc.), dal and as dal flour. It is used in preparing snacks, sweets and condiments. Fresh green seeds are also used as a green vegetable. It is an excellent source of protein (20.47%), carbohydrates (62.95%), fat (6.04%), minerals (calcium, phosphorus, iron etc.) and vitamins [1]. It is a superior animal feed and the straw of gram also have good or satisfactory forage value [2]. Due to the presence of malic and oxalic acid, the leaves of chickpea have sour taste. Malic and oxalic acids collected from green leaves of are prescribed for intestinal disorders. Chickpea is used as blood purifier and germinated seeds are recommended to cure scurvy disease.
Chickpea is cultivated in 25 states of India, Rajasthan is one of the major chickpea growing states of India after Madhya Pradesh and Maharashtra. In Rajasthan the major chickpea growing districts are Ajmer, Bikaner, Hanumangarh, Pali, Tonk, Bhilwara, Jaisalmer, Nagaur and Jaipur etc. In India, it occupies an area of 10 million hectares and its production is 11.91 million tons with an average productivity of 1192 kg ha-1. In Rajasthan, it is grown over 2.11 million hectares area with annual production and productivity of 2.27 million tons and 1072 kg ha-1, respectively. Unfortunately, the productivity of chickpea is not at the level of satisfaction i.e., average global productivity of 1800 kg ha-1 and it remains plateau since independence of India [3].
Nearly 172 pathogens (67 fungi, 22 viruses, 3 bacteria, 80 nematodes and phytoplasma) will cause diseases in chickpea [4]. These pathogens may be of bacteria, mycoplasma, fungi, nematodes and viruses which contain high genotypic variation. Among various biotic factors attributed to low productivity of chickpea, susceptibility to diseases is very important. Chickpea is susceptible to different diseases including, Fusarium wilt (Fusarium oxysporum f. sp. ciceri), dry root rot (Rhizoctonia bataticola), wet root rot (Rhizoctonia solani), collar rot (Sclerotium rolfsii), Ascochyta blight and Sclerotinia rot (Sclerotinia sclerotiorum (Lib.) de Bary) [5]. These diseases have been found to cause considerable economic losses to the chickpea growers in India [6]. The extent of losses from 20% to 46% at pod bearing stage of the crop and the incidence of disease varied from 18% to 32% [7].
Amongst different diseases, Sclerotinia rot commonly called as stem rot or white mold caused by Sclerotinia sclerotiorum (Lib.) de Bary is one of the most important disease. The detonative pathogenicity under favorable conditions and the ability of producing sclerotia to withstand adverse conditions allow it to be a successful pathogen [8,9]. It causes significant yield losses whenever, the crop is grown in relatively cool and moist areas favorable for its development [7]. The disease causes total crop failure where chickpea is grown in the same paddock in successive years. Dense crops are likely to be the most severely affected, particularly, under moist condition. This disease has been reported from different parts of India and causing considerable losses [10]. The extent of the damage was reported from 21.3% to 46.7% at pod bearing stage of the crop [11]. In Jammu and Kashmir, the disease incidence varied from 18.7% to 32.2%. The recent observations suggested that the incidence and severity of Sclerotinia rot of chickpea is increasing in the Northern part of India. In Rajasthan, stem rot (S. sclerotiorum) was initially observed at Agricultural Research Station, Umedganj, Kota in the year 1993-1994 and thereafter, it has been appearing regularly in mild to severe form in various parts of the state [12].
The fungicides are the most common tools for controlling plant disease. But they are not feasible for environment, health and it leads to environment hazard. Hence integrated management of the disease by using bio-agents and fungicides is the best alternative [13,14]. Therefore, it is an urgent need to use some bio-agents and fungicides which are effective against Sclerotinia rot disease. Hence, keeping in view the importance of chickpea crop and potential threat of Sclerotinia sclerotiorum in all the chickpea growing areas in the Rajasthan, the present investigation was undertaken to manage of this important disease through bio-agents and fungicides.
A field trial was conducted for the management of Sclerotinia rot of chickpea using bioagents and fungicides in rabi season. Talc based formulations of two fungal and two bacterial bioagent were applied as seed treatment and soil application and fungicides were used as seed treatment. Seed treatment of fungicides and seed and soil treatment of the talc based bioagent formulations was done. In case of control, seeds were sown in pathogen inoculated soil without any bioagents and fungicides. Observations were taken periodically for the disease incidence.
Total 13 treatments including control were carried out using randomized block design having plot size of 4 × 4 m². Each treatment was replicated thrice. The experiment was conducted under artificial soil inoculated conditions. For this purpose, sand sorghum meal inoculum of Sclerotinia sclerotiorum was applied at 70 g per plot and mixed properly on top surface soil using a hand rack. Standard agronomic practices recommended for cultivation of chickpea crop in this region were followed. In case of control, the untreated seeds were sown. Observations on disease incidence were recorded periodically as well as the grain yield was recorded after harvesting of the crop.
Incidence of the disease and percent disease control was calculated by using the following formula [15];


The data of percent disease incidence in all the experiments were transformed to their arc sin values [16]. The statistical analysis of the data of all the laboratory experiments was done following completely randomized design. The data of field experiments were analyzed following randomized block design.
The results in Tables 1 and 2, show that Sclerotinia rot incidence in chickpeas was significantly reduced and yield increased when chemical and biocontrol agents were used together, compared to using either chemicals alone or biocontrol agents alone. Among the different treatments, the highest percent of disease control (81.40%) was found in T12-seed treatment with tebuconazole 2% DS at 1.5 g/kg seed+soil application at 10 kg/ha with Trichoderma harzianum followed by T11-ST with tebuconazole 2% DS at 1.5 g/kg seed+SA at 10 kg/ha with Trichoderma viride (80.33%), T10-ST with carbendazim 12%+mancozeb 63% WP at 2 g/kg seed+SA at 10 kg/ha with Trichoderma harzianum (78.89%) and T9-ST with carbendazim 12%+mancozeb 63% WP at 2 g/kg seed+SA at 10 kg/ha with Trichoderma viride (77.45%) as compared to control where 62.37 percent disease incidence was recorded. The highest disease incidence was found in T8-ST with thiophanate methyl 70% WP at 2 g/kg seed (37.77%), followed by T4-ST at 10 g/kg+SA at 10 kg/ha with Bacillus subtilis (34.67%) and T3-ST at 10 g/kg+SA at 10 kg/ha with Pseudomonas fluorescens (32.50%). The results revealed that all twelve treatments, including single fungicides and their combinations with biocontrol agents, were superior to the control in reducing disease incidence and increasing seed yield of chickpea (Figure 1).
| Treatments | Disease incidence (%)* | Disease control (%) |
|---|---|---|
| T1-ST at 10 g/kg+SA at 10 kg/ha with T. viride | 21.33 (27.42) | 65.79 |
| T2-ST at 10 g/kg+SA at 10 kg/ha with T. harzianum | 20.27 (26.70) | 67.5 |
| T3-ST at 10 g/kg+SA at 10 kg/ha with P. fluroescens | 32.50 (34.73) | 47.89 |
| T4-ST at 10 g/kg+SA at 10 kg/ha with B. subtilis | 34.67 (36.04) | 44.41 |
| T5-ST with carbendazim 12%+mancozeb 63% WP at 2 g/kg seed | 26.37 (30.87) | 57.72 |
| T6-ST with tebuconazole 2% DS at 1.5 g/kg seed | 24.23 (29.46) | 61.14 |
| T7-ST with tebuconazole 50%+trifloxystrobin 25% WG at 1.5 g/kg seed | 27.73 (31.74) | 55.53 |
| T8-ST with thiophanate methyl 70% WP at 2 g/kg seed | 37.77 (37.89) | 39.44 |
| T9-T5+SA at 10 kg/ha with T. viride | 14.07 (21.98) | 77.45 |
| T10-T5+SA at 10 kg/ha with T. harzianum | 13.17 (21.23) | 78.89 |
| T11-T6+SA at 10 kg/ha with T. viride | 12.27 (20.46) | 80.33 |
| T12-T6+SA at 10 kg/ha with T. harzianum | 11.60 (19.79) | 81.4 |
| Control | 62.37 (52.15) | 0 |
| S.Em± | 1.66 | |
| CD at 0.05 | 4.87 | |
| CV (%) | 11.04 | |
Note: ST: Seed Treatment; SA: Soil Application; WP: Wettable Powder; WG: Water-Dispersible Granule; DS: Dustable Sulfur; S.Em: Standard Error of the mean; CD: Critical Difference; CV: Coefficient of Variation.
Table 1: Management of Sclerotinia rot of chickpea by bioagents and fungicides.
| Treatments | Yield (q ha-1)* | Yield increase over control (%) |
|---|---|---|
| T1-ST at 10g/kg+SA at 10 kg/ha with T. viride | 12.03 (20.22) | 124.22 |
| T2-ST at 10g/kg+SA at 10 kg/ha with T. harzianum | 12.83 (20.93) | 139.13 |
| T3-ST at 10g/kg+SA at 10 kg/ha with P. fluroescens | 8.90 (17.25) | 65.84 |
| T4-ST at 10g/kg+SA at 10 kg/ha with B. subtilis | 7.93 (16.16) | 47.83 |
| T5-ST with carbendazim 12%+mancozeb 63% WP at 2 g/kg seed | 10.43 (18.69) | 94.41 |
| T6-ST with tebuconazole 2% DS at 1.5 g/kg seed | 11.17 (19.41) | 108.07 |
| T7-ST with tebuconazole 50%+trifloxystrobin 25% WG at 1.5 g/kg seed | 9.70 (18.03) | 80.75 |
| T8-ST with thiophanate methyl 70% WP at 2 g/kg seed | 7.13 (15.33) | 32.92 |
| T9-T5+SA at 10 kg/ha with T. viride | 14.47 (22.28) | 169.57 |
| T10-T5+SA at 10 kg/ha with T. harzianum | 15.37 (23.01) | 186.34 |
| T11-T6+SA at 10 kg/ha with T. viride | 16.07 (23.57) | 199.38 |
| T12-T6+SA at 10 kg/ha with T. harzianum | 16.90 (24.20) | 214.91 |
| Control | 5.37 (13.14) | 0 |
| S.Em± | 1.24 | |
| CD at 0.05 | 3.65 | |
| CV (%) | 11.11 | |
Note: ST: Seed Treatment; SA: Soil Application; WP: Wettable Powder; WG: Water-Dispersible Granule; DS: Dustable Sulfur; S.Em: Standard Error of the mean; CD: Critical Difference; CV: Coefficient of Variation.
Table 2: Effect of bioagents and fungicides on the yield of chickpea.
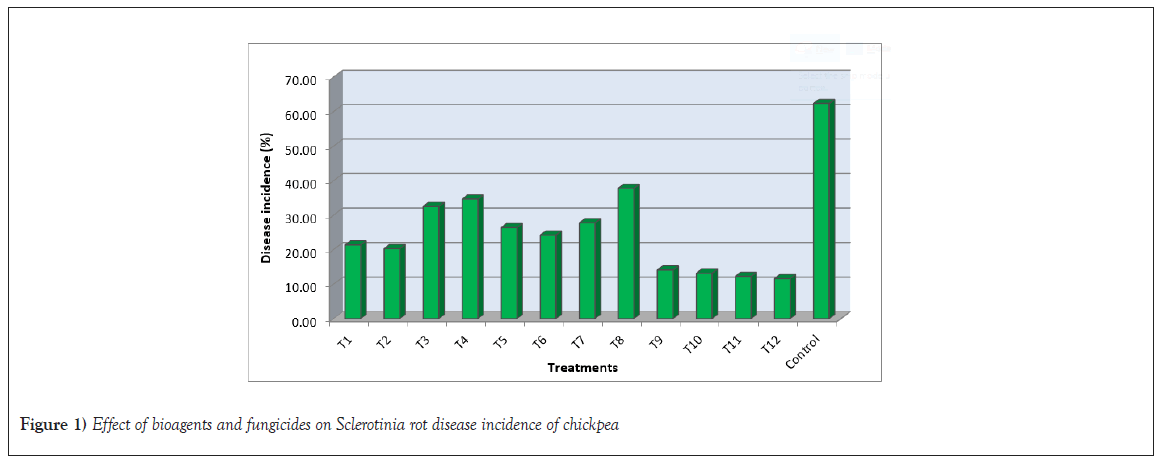
Figure 1: Effect of bioagents and fungicides on Sclerotinia rot disease incidence of chickpea.
Similarly, Pandey et al., found that applying vermicompost at 5.0 tons/ha combined with T. harzianum at 5.0 kg/ha was the most effective treatment, reducing disease incidence by 72.73% and increasing grain yield by 39.69% [17]. This was followed by Farmyard Manure (FYM) at 2.5 tons/ha combined with T. harzianum at 5.0 kg/ha, which resulted in a 64.85% reduction in disease incidence and a 38.17% increase in grain yield. The least reduction in disease incidence (14.55%) and the smallest increase in grain yield (15.27%) were observed with foliar spray of P. fluorescens at 5.0 g/liter. Mandal et al., reported that seed treatment with Pusa 5SD (T. viride) combined with carboxin and Mesorhizobium ciceri, along with a foliar spray of iprodion 40 days after sowing, provided the highest disease reduction and improved plant health parameters such as seed germination, shoot and root lengths and the number of pods per plant [10]. While Pusa Biopellet 4G and Pusa 5SD individually were effective in reducing disease incidence and enhancing plant health, their combined application was superior to using any of these formulations alone. Sharma et al., evaluated six different fungicides against S. sclerotiorum under in vitro conditions and tested their efficacy in controlling Sclerotinia rot in pot conditions [18-20]. Carbendazim and the consortium formulation of carbendazim 12%+mancozeb 63% were significantly superior to the other fungicides in inhibiting the mycelial growth of the pathogen and controlling Sclerotinia rot, achieving 100% growth inhibition at a lower concentration (50 ppm) and 73.94% and 67.57% control of rot incidence when applied as a seed treatment (2 g/kg) and foliar spray (0.1% and 0.2% solution), respectively. Propiconazole was the next most effective, providing 67.41% inhibition of growth at the lowest concentration (50 ppm) and 61.21% protection against rot incidence when applied as a seed treatment (2 ml/kg) and foliar spray (0.1%).
Chickpea is an annual legume crop legume crop cultivated in temperate and subtropical regions. It is a member of the Fabaceae family. Among various diseases, Sclerotinia rot, also known as stem rot or white mold, caused by Sclerotinia sclerotiorum (Lib.) de Bary, is one of the most significant. This disease is highly pathogenic under favorable conditions and produces sclerotia that can endure adverse conditions, making it a highly successful pathogen. It causes substantial yield losses, especially in cool and moist areas conducive to its development. The fungus's explosive pathogenicity and the resilience of its sclerotia contribute to its success as a pathogen. Due to its strict soil-borne nature and wide host range, managing this disease through host resistance or chemical management alone is challenging. Fungicides are commonly used to control plant diseases, but they pose environmental and health risks and can lead to environmental hazards. Therefore, integrated disease management using both bio-agents and fungicides is the best alternative.
[Crossref] [Google Scholar] [PubMed]
Citation: Kumar V. Integrated disease management of sclerotinia rot of chickpea (Cicer arietinum) incited by Sclerotinia sclerotiorum (Lib.) de Bary. AGBIR.2024;40(5):1318-1321.
Received: 12-Aug-2024, Manuscript No. AGBIR-24-145135; , Pre QC No. AGBIR-24-145135 (PQ); Editor assigned: 14-Aug-2024, Pre QC No. AGBIR-24-145135 (PQ); Reviewed: 28-Aug-2024, QC No. AGBIR-24-145135; Revised: 04-Sep-2024, Manuscript No. AGBIR-24-145135 (R); Published: 12-Sep-2024, DOI: 10.35248/0970-1907.24.40.1318-1321
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
