Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2025) Volume 41, Issue 2
Anethum graveolens L. commonly known as dill, is a famous aromatic herb that is widely used as a spice and has been applied in folk medicine to cure many diseases. In this study, we aim to develop the antioxidant activity of the flavonoids extracted using organic solvents: Anti-DPPH test and reducing power assay. The results demonstrate an interesting antioxidant activity which was directly related to the quantities of polyphenols and flavonoids present in the extract. Anethum graveolens L. can be considered as a source of polyphenols.
Dill; Anti-DPPH; Reducing power
The Name of genus Anethum is derived from the Greek word, aneeton or aneeson that means strong-smelling [1]. Anethum graveolens L., (A. graveolens) also known as dill, is a plant native to the Mediterranean area is an aromatic herb, belonging to Apiaceae (Umbelliferae) family, which is distributed in Southwest Asia and the Mediterranean and is native to Southeast Europe [2-4]. Nowadays, it is cultivated for export in most parts of the world, especially Germany, the Netherlands, India, China and the United States (Figure 1) [5].
Figure 1) Dill (Anethum graveolens L.): Flowering and fruiting stem with separate root, flower and fruit.
Dill plant has a long history of use as a culinary and medicinal herb. The leaves are used in salads and soups, while the seeds are drunk as tea and added to sweets [6]. Various pharmacological actions of A. graveolens such as antimicrobial, antispasmodic, anti- diabetic, anti-hypercholesterolaemic and anti-inflammatory have been reported [7]. In addition, some studies have shown that dill seed oil suppresses some spoilage fungi [8,9]. Taken in oral doses drug interactions are reported for anti-diabetic drugs because of cardiovascular effects [10]. On the other hand, A. graveolens was mentioned as ‘‘brain tonic’’ in 17th century in Europe [11]. Recently, it has been reported that it is a potential source of antioxidant and also has anti microbial properties [12].
The phytochemical studies carried out on A. graveolens plant revealed the presence of large number of phytoconstituents such as tannins, flavonoids, triterpenes, coumarins, phenolic acids, capric acid, palmitic acid, stearic acid, oleic acid etc [13,14].
The purpose of this study is to evaluate the antioxidant activity of the flavonoids extracted using organic solvent.
Plant material
The random sampling was used during the harvesting. The areal parts of Anethum graveolens L., were taken from spontaneous plants growth near the station of Setif of National Institute of Agricultural Research. Determined in laboratory by Doctor Wafa Nouioua.
The extraction method
Briefly, 10 g of the dried plant was powdered and macerated in 80 % methanol for 24, 48 and 72 hours, at the laboratory conditions (1:10 w/v), then defatted three times with petroleum ether.
The extract was pooled and concentrated in vacuum (10 mL), which was extracted with chloroform, then acidified with 20% H2SO4 (pH=5) and extracted with ethyl acetate three time. The appearance of an interphase precipitate was observed upon extraction with ethyl acetate. The ethyl acetate fractions were taken as flavonoids for our experiment [15].
Determination of total phenolic content
In polyphenol determination, Folin-Ciocalteu method was used [16]. The samples (0.2 mL) were mixed with 1 mL of the Folin-Ciocalteu reagent (1/10 of dilution). The solutions were allowed to stand for 4 minutes, then, 0.2 mL of a saturated sodium carbonate solution (75 mg/mL) was added. The mixed solutions were allowed to stand for another 120 minutes. The absorbance was measured at 765 nm. Gallic acid was used as a standard for the calibration curve. The total phenolic compounds content was expressed as mg equivalent of gallic acid per gram of extract (mg EAG/GE).
Determination of total flavonoids content
The flavonoids content was estimated according to the method of aluminium chloride solution [17]. Briefly, 1 mL of the methanol solution of the extract was added to 1 mL of 2% AlCl3 in methanol. After 10 minutes, the absorbance was determined at 430 nm. Quercetin was used as a standard. Results were expressed as mg equivalent Quercetin per gram of extract (mg EQ/GE).
DPPH assay
One milliliter of the extract at different concentrations was added to 0.5 mL of a DPPH methanol solution. The mixtures were shaken vigorously and left standing at the laboratory temperature for 30 minutes in the dark. The absorbances of the resulting solutions were then measured at 517 nm.
The antiradical activity was expressed as IC50 (micrograms per milliliter). The ability to scavenge the DPPH radical was calculated using the following equation:
DPPH scavenging effect (%)=[(A0-A1)/A0]
Where:
A0: The absorbance of the control at 30 minutes.
A1: Is the absorbance of the sample at 30 minutes. BHT was used as standard [18].
Reducing power
The reducing power activity was determined according to the method of Oyaizu [18]. 2,5 mL of the extract were mixed with 2.5 mL of sodium phosphate buffer (pH 6.6; 200 mmol/L) and 2.5 mL of potassium ferricyanide (10 mg/mL). The mixtures were incubated at 50°C for 20 minutes. After cooling, 2.5 mL of trichloroacetic acid (100 mg/mL) were added; the mixtures were centrifuged at 200 g for 10 minutes. The upper layer (5 mL) was mixed with 5 mL of deionized water and 1 mL of 1 mg/mL ferric chloride, and the absorbance was measured at 700 nm against a blank. EC50 value (mg extract/ mL) was obtained by interpolation from linear regression analysis. Ascorbic acid was used as a reference standard [19].
Statistical analysis
Results were expressed as the mean ± standard deviation. Data was statistically analyzed using t test of Student as primary test followed by Fisher test with the criterion of P<0.05 to determine whether there were any significant differences between the extract of A. graveolens and standards, using Graph pad prism 5 Demo Software.
The yield, polyphenols and flavonoids estimated quantities were 5.4 %, 28, 24 ± 0,46 mg EAG/GE and 20,63 ± 1,84 mg EQ/GE.
The antioxidant activity of A. graveolens flavonoids crude extract was assessed by spectrophotometry of the presence of the DPPH radical, which is often used to compare the activity of plant extracts (Figure 2). DPPH is a stable free radical which dissolves in methanol and shows characteristic absorption at 517 nm. When an antioxidant scavenges free radicals by hydrogen donation, the DPPH assay solution becomes lighter in color [20].
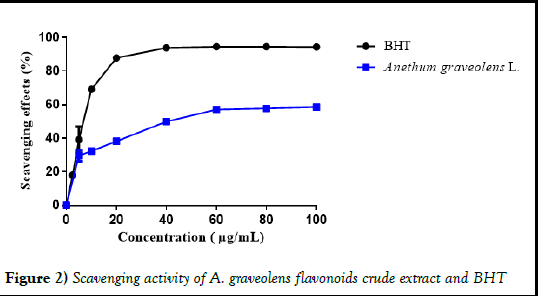
Figure 2) Scavenging activity of A. graveolens flavonoids crude extract and BHT
The quality of the antioxidants in the extract was determined by the IC50 values, which was 46,82 ± 3,29 μg/mL**** against 8,76 ± 0,69 μg/mL for BHT. On the other hand, the scavenging effect of A. graveolens flavonoids crude extract was up to 58,63 ± 0,24%**** compared to 94.39 ± 0.58% for BHT. Hence, the studied extract demonstrates a medium anti-DPPH activity compared of the used standard.
In reducing power assay, the extract indicate an interesting value of EC50 49,55 ± 0,35 μg/mL**** but still weaker than acid ascorbic which was 8,46 ± 0,09 μg/mL (Figure 3).
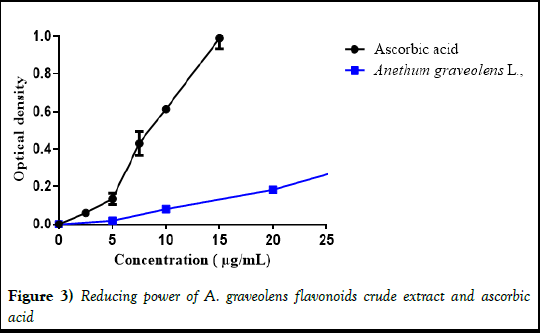
Figure 3) Reducing power of A. graveolens flavonoids crude extract and ascorbic acid.
The antioxidant activity, TPC and TFC of A. graveolens flavonoids crude extract were discussed. The antioxidant potential of the plant might be due to the secondary metabolite found in the extract. Secondary metabolites are natural products that are primarily produced by bacteria, fungi, and plants. They are low-molecular-weight molecules with a range of biological importance, including antioxidants and antimicrobial activity.
Phenolic compounds, as primary antioxidants, work according to two mechanisms: Hydrogen Atom Transfer (HAT) or Single Electron Transfer (SET). The antioxidants that are most effective in stopping free radical chain reactions usually contain an aromatic ring capable of donating H• to free radicals formed during lipid oxidation. The antioxidant radicals thus formed are stabilized by the delocalization of unpaired electrons around the phenol ring to form stable resonance hybrids.
The phenolic hydroxyl groups have a remarkable ability to scavenge free radicals. On the other hand, flavonoids are biologically important compounds with a broad spectrum of biological activities such as antioxidant, anticancer, anti-inflammatory, anti-allergic, anti-angiogenic, and anti-allergic.
Antioxidant compounds cause the reduction of ferric (Fe3+) form to the ferrous (Fe2+) form because of their reductive capabilities. Prussian blue coloured complex is formed by adding FeCl3 to the ferrous (Fe2+) form. Therefore, reduction can be determined by measuring the formation of Perl's Prussian blue at 700 nm.
This study was focused of antioxidant activity of total flavonoids extract from A. graveolens using organic solvents, the anti-DPPH assay demonstrate an important values but still weaker than BHT, which concord with the quantity of polyphenols and flavonoids found.
There is no conflict of interest in this research.
The authors want to thanks RACHEDI Hamza to his help.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Received: 22-Oct-2023, Manuscript No. AGBIR-23-117929; , Pre QC No. AGBIR-23-117929 (PQ); Editor assigned: 24-Oct-2023, Pre QC No. AGBIR-23-117929 (PQ); Reviewed: 07-Nov-2023, QC No. AGBIR-23-117929; Revised: 11-Mar-2025, Manuscript No. AGBIR-23-117929 (R); Published: 18-Mar-2025, DOI: 10.37532/0970-1907.25.41(2).1-3
