Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2023) Volume 39, Issue 6
To observe how various photoperiod regimes and culture media affect in vitro cultures, particularly with regard to direct shoot growth, callus induction and organogenesis. A variety of six cultural media (MS medium, Nitsch and Nitsch, WPM, Schenk and Hildebrant medium, White's medium, and Khudson Solution C) and photoperiod regimes (16:8, 14:10, 12:12, and 8:16) were used to plant the nodal segment, shoot apex, and leaf explant of the Sindhuri cultivar of pomegranate. The leaf explant supplemented with the most responsive level (1.0 mg/l BAP+1.0 mg/l NAA) showed the largest induction of callus. In shoot apex explants treated with WPM at the most responsive dosage (2.5 mg/l BAP), maximum shoot bud induction was also noted. For de novo shoot regeneration from callus culture, WPM supplemented with 1.0 mg/l BAP+2.0 mg/l NAA was shown to be the most effective. WPM and MS media were determined to be the most effective among all the media for callus induction, shoot bud proliferation, and de novo shoot formation. All the photoperiod regimes investigated, the 14:10 hour regime was shown to be the most effective for shoot bud induction, callus differentiation, and de novo shoot development.
Organogenesis; Callus; Culture media; Photoperiod regimes; Micropropagation
The pomegranate (Punica granatum L.) is often recognized under the Punicaceae family, which consists of two species Punica granatum and P. protopunica and a single genus, Punica [1]. Because of its delicious fruits, medicinal and ornamental uses, and global distribution, it is a species of great commercial importance [2].
Although it takes a year to develop new plants, pomegranates are grown vegetatively by root cuttings of hard wood. Fruit trees that are micro propagated can produce true-to-type plants, overcome the challenges of vegetative propagation, and produce planting materials quickly and in large quantities [3].
Pomegranate plants can be propagated via in vitro culture, which is advantageous if we want to make sure the offspring are disease-free and genetically identical to the parent plant. An essential component of pomegranate (Punica granatum) research and growth was in vitro cultivation. The term "in vitro culture" describes the cultivation of plant tissues, cells, or organs under highly controlled conditions, usually in a lab. In situations when more conventional techniques, such as using seeds or cuttings, may not be appropriate, this method is useful for growing uniform, disease-free plants.
Like micro propagation of any other plant species, pomegranate micro propagation depends critically on the culture material used. Culture media are essential for supplying the nutrients and growth elements required for plant tissues to grow in vitro. Initial stages of micro propagation are similarly influenced by photoperiod in terms of callus induction. In order to promote callus production, pomegranate explants, such as shoot tips or nodal segments, are usually cultivated in the dark during the first stage. The photoperiod can be changed to encourage shoot elongation and proliferation once callus has formed.
Changing the photoperiod is a common step in pomegranate micro propagation techniques to encourage shoot proliferation. Extended photoperiods are often well-received by pomegranate shoots, as they replicate the natural growth circumstances of the plant. Consequently, the goal of this study is to determine how different culture media and photoperiod regimes affect the growth of shoots and the induction of callus in the pomegranate variety 'Sindhuri'.
Plant material
The research on Punica granatum cv. Sindhuri was carried out in the Plant Tissue Culture Laboratory of the Department of Plant Breeding and Genetics at S.K.N. College of Agriculture, Jobner. Nodal segments, leaves and shoot apexes were utilized as explants.
Sterilization and preparation of explant
It is challenging to produce sterilized explants because, during the sterilization process, living material should not lose its biological activity-only bacterial and fungal contaminants should be removed. Various surface sterilization treatments were used to sterilize each explant. After properly washing the explants for 20 minutes under running water, they were shaken vigorously for 10 minutes and then again with liquid detergent (RanKleen). Explants were cleaned with detergent and then rinsed for five minutes under running tap water to get rid of any remaining detergent. Lastly, explants were placed in a laminar air flow cabinet and surface sterilized with 0.1 percent HgCl2. Sterilize the leaves for one to two minutes, the apex for three to four minutes and nodal segment for ten to thirteen minutes. After giving them a complete four to five rounds of washing in sterile double-distilled water, these were inoculated into culture media that had been enhanced with different amounts of plant growth regulators.
Inoculation of the explant
The explants were aseptically inoculated onto culture media following sterilization. Using sterile forceps and stringent aseptic conditions, explants were moved to big sterile glass Petri plates for inoculation. Here, a sterile scalpel blade was used to further trim the explants to the appropriate diameters. Explants were clipped to the appropriate size and then vertically moved to borosil flasks, phyta jars, and culture test tubes that contained MS media supplemented with various plant growth regulators. After vertically inoculating the explants in culture, the mouth of phyta jars, test tubes and borosil flasks were quickly flamed than test tubes and borosil flasks were closed with non-adsorbent cotton plug and phyta jars with cap.
Media's impact
The direct shoot proliferation, callus induction, and organogenesis at the most responsive level of plant growth regulators were investigated using a variety of media, including Murashige and Skoog, Nitsch and Nitsch, Woody Plant Medium, Schenk and Hildebrant Medium, White's Medium, and Khudson Solution-C.
Photoperiod's impact
The following photoperiod regimes (16:8, 14:10, 12:12 and 8:16) were evaluated on responsive cultures to observe the impact of various photoperiods on in vitro cultures, particularly with regard to direct shoot growth, callus induction, and organogenesis.
To ensure unbiased results, the entire experiment was repeated twice and each treatment combination was replicated ten times. The study employed a fully randomized design, and the data were subjected to standard error and mean analysis in accordance with Snedecor et al. [3] methodology. Following the value transformation of the shoot induction from explants, the square root transformation was applied to each replication's result to determine the standard error for the number of shoots as follows: -
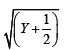
Where, Y=original value
Influence of cultural media on callus induction, shoot proliferation and regeneration
Six different types of culture media-MS medium, Nitsch and Nitsch, WPM, Schenk and Hildebrant medium, White's medium, and Khudson Solution-C were primarily utilized to examine the effects of various culture media on the induction of callus, shoot proliferation, and regeneration. To induce callus in leaf explants, the most responsive level of plant growth regulator (1.0 mg/l BAP+1.0 mg/l NAA) was added to various culture conditions.
In the Woody plant media, the leaf explant showed the most callus (Figure 1). Even at the highest level of response for the plant growth regulator (1.0 mg/l BAP+1.0 mg/l NAA), the Schenk and Hildebrant medium failed to cause callus on the cut ends of the leaves.
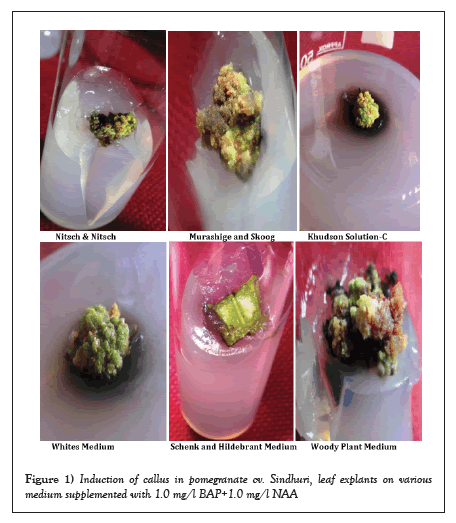
Figure 1: Induction of callus in pomegranate cv. Sindhuri, leaf explants on various medium supplemented with 1.0 mg/l BAP+1.0 mg/l NAA.
An examination of Table 1 additionally demonstrated that 100% of callus induction occurred on the MS, WPM, and White's medium supplemented with 1.0 mg/l BAP+1.0 mg/l NAA. The Khudson Solution C medium showed the lowest frequency of callus induction.
| S. No | Media | Days taken for callus initiation | Colour of callus | Texture of callus | Morphogenetic response (%) | Fresh weight (mg) |
|---|---|---|---|---|---|---|
| 1 | Murashige and skoog medium | 24.2 | Greenish yellow | Semi compact | 100 | 890 |
| 2 | Woody plant medium | 22.3 | Brownish with light green | Semi compact | 100 | 1022 |
| 3 | Nitsch and nitsch medium | 30.8 | Green | Compact | 50 | 550 |
| 4 | Schenk and hildebrant medium | - | - | - | - | - |
| 5 | White’s medium | 26.5 | Green | Compact | 100 | 670 |
| 6 | Khudson solution-C | 35.1 | Light green | Compact | 40 | 438 |
Table 1: Impact of 1.0 mg/l BAP and 1.0 mg/l NAA on the production of callus in pomegranate cv. Sindhuri leaf explants on various culture media.
After 12 to 25 days of incubation, the shoot apex explant that had been inoculated on various culture media began to produce shoot buds in the explant. Maximum shoot bud induction was observed on WPM followed by MS media supplemented with most responsive level of PGR (2.5 mg/l BAP) for shoot bud induction with 100 percent frequency (Table 2 and Figure 2). Schenk and Hildebrant and Khudson Solution-C media did not induce any shoot bud in shoot apex explant on responsive levels except, further elongation of shoot apex.
| S. No | Media | Days taken for shoot bud initiation | Number of shoot bud induced | Shoot length (cm) | Morphogenetic response (%) |
|---|---|---|---|---|---|
| 1 | Murashige and skoog medium | 14.5 | 1.7# (2.4) | 2.64# (6.48) | 100 |
| 2 | Woody plant medium | 12.8 | 1.75# (2.6) | 2.67# (6.64) | 100 |
| 3 | Nitsch and nitsch medium | 25.3 | 0.93# (0.6) | 1.26# (1.84) | 30 |
| 4 | Schenk and hildebrant medium | - | 0.70# (-) | 0.70# (-) | - |
| 5 | White’s medium | 20 | 1.26# (1.3) | 1.88# (3.95) | 60 |
| 6 | Khudson solution-C | - | 0.70# (-) | 0.70# (-) | - |
| Mean sum of squares due to treatment | 2.24** | 7.93** | |||
| Mean sum of squares due to error | 0.08 | 0.32 | |||
| CD at 5% | 0.39 | 0.51 | |||
Note: ** Significant at p=0.01, #=Transformed values, (-)=No response, ()=Value in parenthesis represents mean de novo developed shoots.
Table 2: Impact of 2.5 mg/l BAP on the induction of shoot buds in the shoot apex explant of the pomegranate cultivar Sindhuri on various culture mediums.

Figure 2: Induction of shoot bud in shoot apex explant of pomegranate cv. Sindhuri on various culture media supplemented with 2.5 mg/l BAP supplement.
Furthermore, the analysis of Table 2 showed that shoot bud induction and shoot length were significantly variable between cultural mediums. Maximum shoot bud induction and shoot length were noted in WPM; however, there was no statistically significant difference when compared to MS medium.
Within 25 to 38 days of incubation, leaf-derived callus (1.0 mg/l BAP+1.0 mg/l NAA) was subculture on various media supplemented with 1.0 mg/l BAP+2.0 mg/l NAA to produce de novo shoots in MS medium, Nitsch & Nitsch, WPM, and White's medium. The WPM was used to observe the maximum de novo shoot bud induction from callus culture, followed by MS media supplemented with 1.0 mg/l BAP+2.0 mg/l NAA (Figure 3).

Figure 3: Regeneration on various media supplemented with 1.0 mg/l BAP+2.0 mg/l NAA in callus generated from leaves (1.0 mg/l BAP+1.0 mg/l NAA).
Even with plant growth regulators present that responded to other medium, callus did not show signs of shoot morphogenesis when cultivated on Khudson Solution-C. Similar to shoot bud induction, the de novo shoot development maximized in WPM; however, there was no statistically significant difference between the two. Regarding de novo shoot regeneration, all other media varied significantly (Table 3).
| S. No | Media | Days taken for shoot bud initiation | Number of de novo shoot bud induction | Morphogenetic response (%) |
|---|---|---|---|---|
| 1 | Murashige and skoog medium | 25.5 | 1.76#(3.4) | 60 |
| 2 | Woody plant medium | 29.9 | 2.11#(4.8) | 70 |
| 3 | Nitsch and nitsch medium | 38.1 | 1.12#(1.2) | 30 |
| 4 | Schenk and hildebrant medium | - | 0.70#(-) | - |
| 5 | White’s Medium | 35.3 | 1.22#(1.4) | 40 |
| 6 | Khudson Solution-C | - | 0.70#(-) | - |
| Mean sum of squares due to treatment | 3.23** | |||
| Mean sum of squares due to error | 0.45 | |||
| CD at 5% | 0.6 | |||
Note: ** Significant at p=0.01, #=Transformed values, (-)=No response, ()=Value in parenthesis represents mean de novo developed shoots.
Table 3: Regeneration in leaf-derived callus on various media supplemented with 1.0 mg/l BAP+2.0 mg/l NAA (1.0 mg/l BAP+1.0 mg/l NAA).
Effect of photoperiod
The physiological reaction of an organism to the duration of day and night is known as photoperiod. Both plants and animals experience it. Another way to describe photoperiodism is as a plant's developmental reactions to day and night length. The fact that photoperiodic effects are correlated with the timing of both the light and dark phases.
Using standard callus induction (1.0 mg/l BAP+1.0 mg/l NAA), micro propagation protocol (2.5 mg/l BAP for nodal segment and 2.5 mg/l BAP for shoot apex explant), and regeneration protocol (1.0 mg/l BAP+2.0 mg/l NAA), various photoperiod regimes were evaluated for their morphogenetic effect in pomegranate cv. Sindhuri. Variations in photoperiod regimes (16:8, 14:10, 12:12, and 8:16) were applied to standard protocols.
Maximum callus induction was seen in photoperiod regimes of 16:8 hours from cut ends of leaf explants when they were cultured on MS media supplemented with 1.0 mg/l BAP+1.0 mg/l NAA under various photoperiod regimes. The leaf explant's cut surface did not develop enough callus to cause further swelling during the 8:16 photoperiod (Table 4).
| S. No | Photoperiod regime | Days taken in callus induction | Callus weight (mg) | Morphogenetic response (%) |
|---|---|---|---|---|
| 1 | 16:08 | 26.8 | 639.6 | 100 |
| 2 | 14:10 | 23.4 | 874.9 | 100 |
| 3 | 12:12 | 32.7 | 462.1 | 70 |
| 4 | 08:16 | - | - | - |
Note: -=No callus induction.
Table 4: Various photoperiod regimes' effects on callus induction in leaf explants supplemented with 1.0 mg/l BAP and 1.0 mg/l NAA.
In the current study, shoot apex explants incubated at 14:10 hours photoperiod and then 16:8 hours’ photoperiod showed the largest shoot bud induction (2.3). Even on the sensitive level of plant growth regulators, an 8:16 photoperiod was insufficient to elicit shoot buds in shoot apex explants, with the exception of increased shoot growth. At various photoperiod regimes, a noteworthy variation was noted in the induction of shoot buds from shoot apex explants (Table 5).
| S. No | Photoperiod regime | Number of shoot bud induction | Shoot length (cm) | Morphogenetic response (%) |
|---|---|---|---|---|
| 1 | 16:08 | 1.4# (1.5) | 2.24# (5.12) | 80 |
| 2 | 14:10 | 1.7# (2.3) | 2.60# (6.32) | 100 |
| 3 | 12:12 | 1.1# (0.9) | 1.45# (2.44) | 40 |
| 4 | 08:16 | 0.7# (-) | 0.7# (-) | - |
| Mean sum of squares due to treatment | 4.93** | 12.43** | ||
| Mean sum of squares due to error | 0.07 | 0.26 | ||
| CD at 5% | 0.37 | 0.46 |
Note: ** Significant at p=0.01, #=Transformed values, (-)=No response, ()=Value in parenthesis represents mean de novo developed shoots.
Table 5: Impact of various photoperiod regimes on induction of shoot buds in shoot apex treated with 2.5 mg/l BAP.
Table 6's examination revealed that there were notable variations in the de novo shoot regeneration from callus cultures under various photoperiod conditions. Shortening the dark period improved the reaction, whereas lengthening it had the opposite effect. Organogenesis from callus cultures showed similar responses to various photoperiods, although no regeneration was seen in cultures incubated at 12:12 and 8:16 hours. At various photoperiod regimes, there were notable differences in organogenesis.
| S. No | Photoperiod regime | Days taken in regeneration | Number of de novo regenerated shoots | Morphogenetic response (%) |
|---|---|---|---|---|
| 1 | 16:08 | 35.5 | 1.14# (1.1) | 40 |
| 2 | 14:10 | 30.1 | 1.81# (3.2) | 70 |
| 3 | 12:12 | - | 0.70# (-) | - |
| 4 | 08:16 | - | 0.70# (-) | - |
| Mean sum of squares due to treatment | 4.80** | |||
| Mean sum of squares due to error | 0.15 | |||
| CD at 5% | 0.35 | |||
Note: ** Significant at p=0.01, #=Transformed values, (-)=No response, ()=Value in parenthesis represents mean de novo developed shoots.
Table 6: Impact of photoperiod regimes on callus culture's de novo shoot regeneration.
Effect of media
An essential component influencing the growth and morphogenesis of plant tissues is the growth medium's composition. The components of plant tissue culture medium include carbon sources, organic supplements, stabilizing agents, vitamins, amino acids or other nitrogen supplements, and growth regulators. In plant tissue culture, Murashige et al. [4] is the most often used media. For a large variety of plant species, the B5 [5], N6 [6], and Nitsch and Nitsch [7] have been frequently utilized. Furthermore, the DKW [8] and the WPM medium [9] are utilized for the cultivation of woody species. The growth media is chosen with the plant species and tissue culture in mind [10].
Examining Tables 2 and 3 showed that there were notable variations between the culture media with regard to the number of shoot buds generated per explant, shoot length, morphogenetic response, and de novo shoot development. Woody plant media showed the greatest shoot bud induction and shoot length, followed by MS media. These findings are consistent with El-Agamy et al., [11] conclusion that when pomegranate cultivars are propagated, WPM considerably produces a larger average number of nodes than those grown on MS medium.
Effect of photoperiod
Different benefits result from the capacity to schedule specific developmental stages for times of the year when the environment is most likely to be favorable. For example, if reproduction were timed to occur in the spring, susceptible young offspring would have more time to mature before facing the harsh winter weather. This would increase the likelihood of the offspring surviving. Thus, plants and animals that have developed mechanisms to detect and respond to changes in photoperiod which allows them to sense seasonal differences have an advantage over others. The amount of light and darkness in a 24-hour cycle is known as the photoperiod. Numerous developmental reactions in plants, animals, and even fungus are regulated by photoperiod. Because day length is a good predictor of the season and allows developmental events to be planned to match with certain environmental conditions, the response to photoperiod has changed over time [12].
Plant growth is influenced by a number of variables, including light duration, intensity, and wavelength [13]. While light plays a crucial role in micro propagation, there aren't many studies on how artificial light intensities affect plant development in general and orchid growth in particular. This is mostly due to the difficulty in achieving the increased light intensity needed for certain plants to reach maturity and the area these plants now require. Plant performance likely reflects the photosynthetic mechanism for comparatively short times.
In general, light, duration, temperature, and moisture supply are external elements that affect plant growth and development in addition to internal factors like genotype and plant hormones. This outcome could be the consequence of internal variables that directly impact plant development interacting with light intensity. The optimal product outcome will come from appropriate light intensity and duration [14].
The physiological response of organisms to the duration of day or night is known as photoperiodism. The developmental reactions of plants to the relative durations of the light and dark phases is another definition of photoperiodism. Therefore, it's important to stress that photoperiodic effects are directly related to when the light and dark periods occur.
Using MS media supplemented with varying responsive levels of plant growth regulators, various photoperiod regimes (16:8, 14:10, 12:12, and 8:16) were evaluated for shoot bud, callus induction, and de novo shoot regeneration in the current study. Maximum callus growth, shoot bud induction, and de novo shoot regeneration were noted at photoperiods of 14:10 and 16:8 hours, respectively. Similar findings were also noted by Jakhar et al., [15], Kumawat [16] in Aloe vera, Nagar [17] and Burdak et al., [18] in fenugreek, Kumar and Jakhar [19], Kumar et al., [20], Kumar and Jakhar [21], and Kumar [22] in pomegranate. The shortest light hour (8:16) was insufficient to induce shoot buds, regenerate de novo shoots, and promote callus growth in leaves. These results were also in agreement with the Gliricidia research conducted by Choudhary et al., [23] and Aparna et al., [24].
Zakizadeh et al., [25] found that there were notable variations in bulblets diameter, leaf length, and shoot length in Amaryllis plants grown under different photoperiods. Compared to photoperiod regimes of 12:12 hours, the leaf length increased under 16:8 and 14:10 hours. There were no appreciable variations between the 16:8 and 14:10 photoperiods. The results of the current study showed that the highest shot length was likewise recorded for photoperiods of 16:8 and 14:10. Nonetheless, in the current study, there were notable variations at different photoperiod regimes, suggesting that the kind of plant and the concentration of plant growth regulators have an essential influence in in vitro conditions.
These findings, however, differed significantly from those of Burger et al., [26] in Rosa hybrida and Sherawat et al., [27] in Rauwolfia serpentine. Goyal et al., [28] in Ber, Aasim et al., [29] in Urginea maritime, Tyagi et al., [30] in Ginger, and Gurjar [31] in Aloe vera all found that a 16:8-hour photoperiod improved regeneration. This could be because different types of plants and explants are used. The current study found that, in vitro, the medium treated with varying levels of responsive plant growth regulators varied significantly according on photoperiod.
In present research investigation, the leaf explant supplemented with the most responsive level (1.0 mg/l BAP+1.0 mg/l NAA) showed the largest induction of callus. In shoot apex explants treated with WPM at the most responsive dosage (2.5 mg/l BAP), maximum shoot bud induction was also noted. For de novo shoot regeneration from callus culture, WPM supplemented with 1.0 mg/l BAP+2.0 mg/l NAA was shown to be the most effective. WPM and MS media were determined to be the most effective among all the media for callus induction, shoot bud proliferation, and de novo shoot formation. All the photoperiod regimes investigated, the 14:10 hour regime was shown to be the most effective for shoot bud induction, callus differentiation, and de novo shoot development.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Kumar R. Effect of culture media and photoperiod on in vitro culture of pomegranate cv. Sindhuri. AGBIR.2023;39(6):748-754.
Received: 01-Nov-2023, Manuscript No. AGBIR-23-122048; , Pre QC No. AGBIR-23-122048 (PQ); Editor assigned: 03-Nov-2023, Pre QC No. AGBIR-23-122048 (PQ); Reviewed: 22-Nov-2023, QC No. AGBIR-23-122048; Revised: 29-Nov-2023, Manuscript No. AGBIR-23-122048 (R); Published: 06-Dec-2023, DOI: 10.35248/0970-1907.23.39.748-754
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
