Agricultural and Biological Research
RNI # 24/103/2012-R1
Research - (2023) Volume 39, Issue 3
Plants have a variety of fungal communities on their surfaces (exophytic) and within their tissues (endophytic). They are essential for plant health and the environment. There is an unexplored diversity of fungal associations with medicinal plants. Catharanthus roseus, a medicinal plant, plays a critical role in global health care. A total of 153 fungal isolates were isolated from thirty leaves of the C. roseus plant, including 11 endophytic and 142 exophytic isolates. Based on morphological characteristics, the isolates were assigned to six endophytic fungi genera and 11 species, as well as 12 exophytic fungi genera and 33 species. Endophytes have significantly less fungal diversity compared to epiphytes. By sequencing the ITS region of rDNA, five epiphytic and three endophytic fungal species were identified molecularly. The isolates were Aspergillus caespitosus, A. elegans, A. flavus, A. nidulans, Penicillium chrysogenum, P. commune and Talaromyces purpurogenus. Aspergillus was the most abundant fungal genus isolated, with four endophytic species out of eleven and nine exophytic species out of thirty-three. A. niger2 was the most common exophytic species, with 28 isolates, an 18.6% colonization frequency, and a relative frequency of 19.7%. In addition, A. caespitosus was the most common endophyte, accounting for 1.3% of the CF and 18.18% of the RF. In this study, we aim to compare epiphytes and endophytes diversity that performed poorly on medicinal plants. This discovery can provide new perspectives and opportunities for compounds and mycotoxins with medicinal value.
Endophytic fungi; Exophytic fungi; Catharanthus roseus; Medicinal plants
The interaction between plant and microbial communities is responsible for the maintenance of biodiversity, ecosystem functioning, and community stability [1]. Most plant species have diverse microbial endo and exophytes community, as well as non-pathogenic fungi or bacteria that colonize the interior or surface of plants [2]. Epiphytic fungi are microorganisms that live permanently or casually on the plant’s surface [3]. Phylloplane or exophytic fungi are classified into two types: resident and causal [4]. Resident exophytes may multiply and grow on the surface of healthy leaves without harming the host, whereas casual exophytes land on the healthy leaf surface in the form of spores and mycelia but cannot grow like residents [5].
Endophytes are microorganisms that live within plant tissues without causing visible or detectable symptoms [6]. Exophytic and endophytic myco-populations grow only a few millimeters apart and have very different compositions [7]. Exophytes grow on the leaf's surface in contact with the outer environment, whereas endophytes are in contact with the inner microenvironment. The coexistence of exophytic and endophytic microorganisms may be important for plant health and protection, as well as microbial biodiversity (Hawksworth and Rossman, 1997; Andrews and Harris, 2000) [8,9]. People have been using plants as natural medicine since ancient times [10].
Catharanthus roseus (L.) G. Don is an Apocynaceae family member and one of the most studied short-lived medicinal plant species, also known as Madagascar Periwinkle (MP) [11]. This plant species is native to Madagascar and is commonly grown as an ornamental plant in parks, gardens, and farms to improve the appearance of landscape areas [12]. An important medicinal plant is critical to global health care. Apart from being the most important natural drug source, MP is also one of the most important model organisms for studying plant alkaloid metabolites due to its ability to synthesize a diverse range of Terpenoid Indole Alkaloids (TIAs); a large group of compounds that come from plants and have many powerful medical uses, with a broad pharmaceutical spectrum. All plant parts, including roots, leaves, flowers, and stalks, have been used in regional herbal medicine. Alkaloids extracted from the whole dried plant are used in modern medicine [13,14].
Several studies have shown that C. roseus is toxic, even though endophytic and exophytic fungi can survive in this toxic plant due to bioactive compounds and may support plant growth [15,16]. Although, the C. roseus plant is poisonous if taken orally or injected subcutaneously. It is poisonous in grazing animals [17,18]. The purpose of this investigation is to isolate and identify the endophytic and exophytic fungi associated with the C. roseus plant, as well as to determine the microbial diversity. This discovery can provide new perspectives and opportunities for compounds and mycotoxins with medicinal value.
Collection of plant samples
The plant leaves samples were collected from King Abdul-Aziz University in Jeddah, Saudi Arabia. Five samples were replicated three times for both endophytic and exophytic fungi. From March to April 2022, thirty leaf samples were collected from a healthy and mature shrub growing to a height of 50-80 cm at an average temperature of 30°C. Sterilized polythene bags were used to collect the samples. They were delivered to the laboratory, and they were processed within twenty-four hours of being collected.
Isolation of the endophytic and exophytic fungi
Endophytic fungi were isolated from C. roseus leaves using the method described by Yan et al., [19]. Samples of fresh, healthy leaves were collected. Leaf surfaces was first disinfected by immersing them in 70% ethanol for 60 seconds, then 90 seconds in 70% sodium hypochlorite, and finally in sterile distilled water for 60 seconds to remove all chemicals before air-drying for 5 minutes on filter paper (150 mm) under sterilization conditions. To prevent endophytic bacterial growth, disinfected leaf tissues were cut into 1cm × 1cm segments and ten pieces were placed on petri dishes supplemented with lactic acid. At room temperature, the dishes were incubated for 14 days then repeatedly observed for endophytic fungal growth. To obtain a pure culture, an inoculum of each new colony was continuously transferred onto a fresh PDA medium. The same procedure will be used to isolate exophytic fungi, but healthy leaves will be used without washed or sterilized with solutions [20].
Morphological identification of fungal isolates
After incubation at 27°C for 5 days, isolates were identified using the method developed by Samson et al., [21], Bokhari [22], Pitt and Hocking [23]. Macroscopically identifying isolates to determine the colony color and growth rate. While septa in hyphae, spores, as well as sporangiophores are observed under microscope.
Molecular identification of fungal isolates
Seven fungal isolates were selected for molecular identification, including two isolates of the endophytic fungi SM05 and SM10. In addition, five exophytic fungal isolates were labeled SH03, SH20, SH31, SH32, and SH36. The isolates were identified by molecular technique with the Internal Transcribed Spacer (ITS): ITS1 and ITS4 [24,25]. BLAST DNA sequences of the isolates were used for BLAST analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi) using default parameters, and sequencing data were analyzed against the nucleotide collection database. These were then compared using a BLAST search in GenBank. By employing the Jukes-Cantor model as described by Bokhari et al., [26], the phylogenetic tree was inferred. Identification at the taxonomic level was based on ≥ 99% ITS similarity.
Calculation of colonization frequency and relative frequency
Colonization Frequency (CF) is defined by Suryanarayanan et al., [27]. Number of segments colonized by endophytes divided by the total number of segments observed multiplied by 100. Relative Frequency (RF), as defined by Piska et al., [28]. Number of occurrence samples of species divided by the total number of isolated fungi multiplied by 100.
A total of 153 fungal isolates, including11endophytic fungal isolates, and 142 exophytic fungal isolates, were isolated from thirty leaves of the C. roseus plant and identified based on morphological characteristics as belonging to six genera and 10 species for endophytic fungi, and 12 genera and 33 species for exophytic fungi. Using morphological characteristics and ITS sequence analysis, the fungal isolates were identified. Based on toxin production by the TLC method, seven endophytic and exophytic isolates were chosen for molecular identification. To identify the fungi, the sequences were compared to those in the GenBank database. Table 1 lists the identified fungi, along with their respective codes, GenBank accession numbers, and percentage of identity. In addition, we constructed a phylogenetic tree utilizing NCBI ITS 1/5.8S rDNA/ITS 2 sequences. This phylogenetic tree illustrates the evolutionary relationship between the studied fungi strains (Figures 1 and 2a-2c).
| S no. | Culture ID | Fungal type | Species identified | GenBank accession no. | Max. identity (%) |
|---|---|---|---|---|---|
| 1 | SM31 | Exo | Aspergillus caespitosus | OQ506575 | 100% |
| 2 | SM10 | Endo | Aspergillus elegans | OQ506573 | 93.97% |
| 3 | SM03 | Exo | Aspergillus flavus | OQ506578 | 93.81% |
| 4 | SM20 | Exo | Aspergillus nidulans | OQ506576 | 93.50% |
| 5 | SM05 | Endo | Penicillium chrysogenum | OQ506572 | 92.33% |
| 6 | SM36 | Exo | Penicillium commune | OQ506577 | 92.35% |
| 7 | SM32 | Exo | Talaromyces purpurogenus | OQ506579 | 91.61% |
Table 1: Fungal isolates from C. roseus plant leaves.
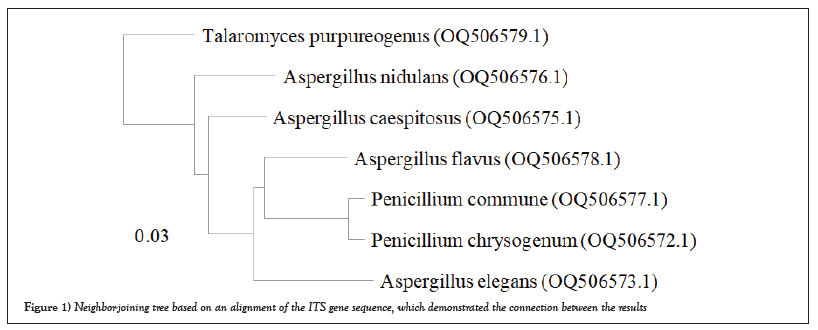
Figure 1: Neighbor-joining tree based on an alignment of the ITS gene sequence, which demonstrated the connection between the results
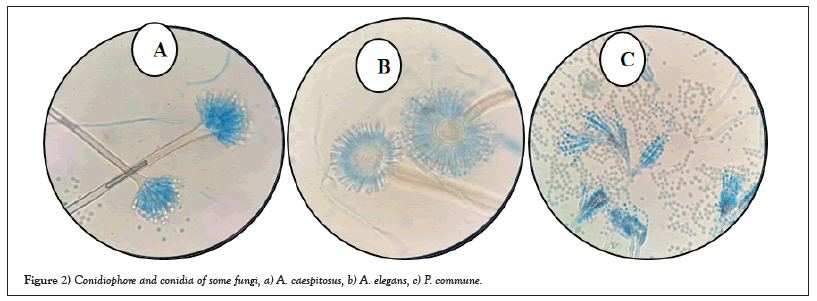
Figure 2: Conidiophore and conidia of some fungi, a) A. caespitosus, b) A. elegans, c) P. commune.
The results in Table 2 showed that the most abundant fungal genus isolated was Aspergillus, with four endophytic species out of eleven and nine exophytic species out of thirty-three. A. caespitosus was the most prevalent endophyte, with CF of 1.3% and RF of 18.18%. Oher’s endophyte species showed the same CF of 0.66% and RF of 9.09 % (Figures 3 and 4) while A. niger 2 was the most prevalent exophytic species. About 28 isolates had a CF of 18.6% and RF of 19.7%, followed by A. niger1. About 19 isolates had CF 12.6% and RF 13.3%, Nigrospora sphaerica, 11 isolates with CF 7.3% and RF 7.7 % while A. flavus, 10 isolates had CF 6.6 % and RF 7%. Aspergillus sp.1 had CF of 4.6% and RF% of 4.9%, whereas Aspergillus sp.3 and Cladosporium macrocarpum had CF% of 4% and RF of 4.2%, respectively. Alternaria spp. 1 and 2 showed CF 3.3% and RF 3.5%, while A. janus and Alternaria sp. 4 showed CF 2.6% and RF 2.8 %. Also, A. caespitosus, Alternaria sp. 3, Aspergillus sp. 2, Curvularia sp. 2, and Talaromyces purpurogenus all have CF 2% and RF 2.1. %. Also, C. phaerospermum, Curvularia sp. 1, Curvularia sp. 3, Mycelia sterilia, and Venturia sp. showed CF 1.3 % and RF 1.4. % The lowest frequencies were shown in Alternaria sp. 5, A. nidulans, C. cucumerinum, C. uredinicola, Curvularia sp. 4, Drechslera avenacea, D. ellisii, D. spicifera, Paecilomyces variotii, P. commune, P. brevicompactum and Ulocladium sp. with CF 0.66 %and RF0.70% (Figures 5 and 6).
| Sample No. | Fungal species | Epiphytes | Endophytes | ||||
|---|---|---|---|---|---|---|---|
| Isolates | %CF | %RF | Isolates | %CF | %RF | ||
| 1 | Alternaria sp.1 | 5 | 3.3 | 3.5 | 0 | 0 | 0 |
| 2 | Alternaria sp.2 | 5 | 3.3 | 3.5 | 0 | 0 | 0 |
| 3 | Alternaria sp.3 | 3 | 2 | 2.1 | 0 | 0 | 0 |
| 4 | Alternaria sp.4 | 4 | 2.6 | 2.8 | 0 | 0 | 0 |
| 5 | Alternaria sp.5 | 1 | 0.66 | 0.7 | 0 | 0 | 0 |
| 6 | Arthrinium sp. | 0 | 0 | 0 | 1 | 0.66 | 9.09 |
| 7 | Aspergillus caespitosus | 3 | 2 | 2.1 | 2 | 1.3 | 18.18 |
| 8 | Aspergillus elegans | 0 | 0 | 0 | 1 | 0.66 | 9.09 |
| 9 | Aspergillus flavus | 10 | 6.6 | 7 | 0 | 0 | 0 |
| 10 | Aspergillus janus | 4 | 2.6 | 2.8 | 0 | 0 | 0 |
| 11 | Aspergillus nidulans | 1 | 0.66 | 0.7 | 0 | 0 | 0 |
| 12 | Aspergillus niger 1 | 19 | 12.6 | 13.3 | 0 | 0 | 0 |
| 13 | Aspergillus niger 2 | 28 | 18.6 | 19.7 | 0 | 0 | 0 |
| 14 | Aspergillus sp.1 | 7 | 4.6 | 4.9 | 0 | 0 | 0 |
| 15 | Aspergillus sp.2 | 3 | 2 | 2.1 | 0 | 0 | 0 |
| 16 | Aspergillus sp.3 | 6 | 4 | 4.2 | 0 | 0 | 0 |
| 17 | Aspergillus versicolor | 0 | 0 | 0 | 1 | 0.66 | 9.09 |
| 18 | Chaetomium globosum | 0 | 0 | 0 | 1 | 0.66 | 9.09 |
| 19 | Chaetomium sp. | 0 | 0 | 0 | 1 | 0.66 | 9.09 |
| 20 | Cladosporium cucumerinum | 1 | 0.66 | 0.7 | 0 | 0 | 0 |
| 21 | Cladosporium macrocarpum | 6 | 4 | 4.2 | 0 | 0 | 0 |
| 22 | Cladosporium phaerospermum | 2 | 1.3 | 1.4 | 0 | 0 | 0 |
| 23 | Cladosporium sp. | 0 | 0 | 0 | 1 | 0.66 | 9.09 |
| 24 | Cladosporium uredinicola | 1 | 0.66 | 0.7 | 0 | 0 | 0 |
| 25 | Curvularia sp.1 | 2 | 1.3 | 1.4 | 0 | 0 | 0 |
| 26 | Curvularia sp.2 | 3 | 2 | 2.1 | 0 | 0 | 0 |
| 27 | Curvularia sp.3 | 2 | 1.3 | 1.4 | 0 | 0 | 0 |
| 28 | Curvularia sp.4 | 1 | 0.66 | 0.7 | 0 | 0 | 0 |
| 29 | Derchslera avenacea | 1 | 0.66 | 0.7 | 0 | 0 | 0 |
| 30 | Drechslera ellisii | 1 | 0.66 | 0.7 | 0 | 0 | 0 |
| 31 | Drechslera spicifera | 1 | 0.66 | 0.7 | 0 | 0 | 0 |
| 32 | Mycelia sterilia | 2 | 1.3 | 1.4 | 0 | 0 | 0 |
| 33 | Nigrospora sphaerica | 11 | 7.3 | 7.7 | 0 | 0 | 0 |
| 34 | Paecilomyces variotii | 1 | 0.66 | 0.7 | 0 | 0 | 0 |
| 35 | Penicillium brevicompactum | 1 | 0.66 | 0.7 | 0 | 0 | 0 |
| 36 | Penicillium chrysogenum | 0 | 0 | 0 | 1 | 0.66 | 9.09 |
| 37 | Penicillium commune | 1 | 0.66 | 0.7 | 0 | 0 | 0 |
| 38 | Penicillium sp. | 0 | 0 | 0 | 1 | 0.66 | 9.09 |
| 39 | Rhizopus sp. | 0 | 0 | 0 | 1 | 0.66 | 9.09 |
| 40 | Talaromyces purpurogenus | 3 | 2 | 2.1 | 0 | 0 | 0 |
| 41 | Ulocladium sp. | 1 | 0.66 | 0.7 | 0 | 0 | 0 |
| 42 | Venturia sp. | 2 | 1.3 | 1.4 | 0 | 0 | 0 |
| Total no. of isolates | 142 | 11 | |||||
Table 2: Colonization frequency and relative frequency (%) of epiphytic and endophytic fungi isolated from C. roseus.
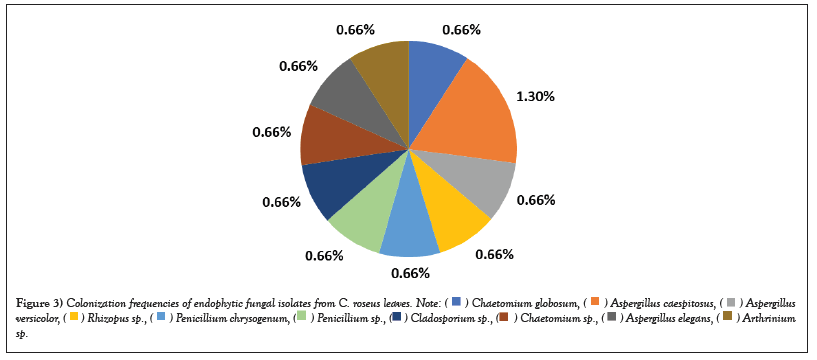
Figure 3: Colonization frequencies of endophytic fungal isolates from C. roseus leaves. 


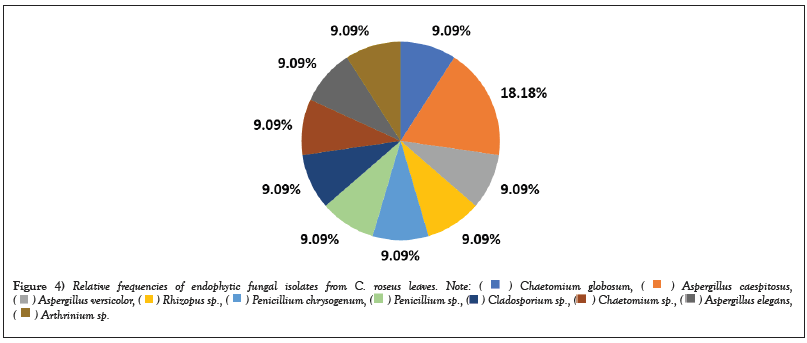
Figure 4: Relative frequencies of endophytic fungal isolates from C. roseus leaves. 


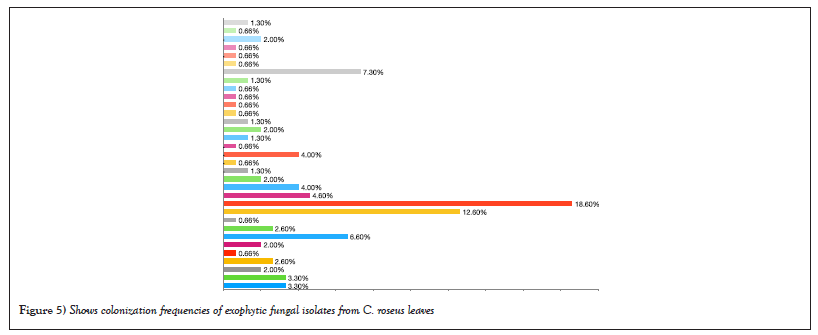
Figure 5: Shows colonization frequencies of exophytic fungal isolates from C. roseus leaves.
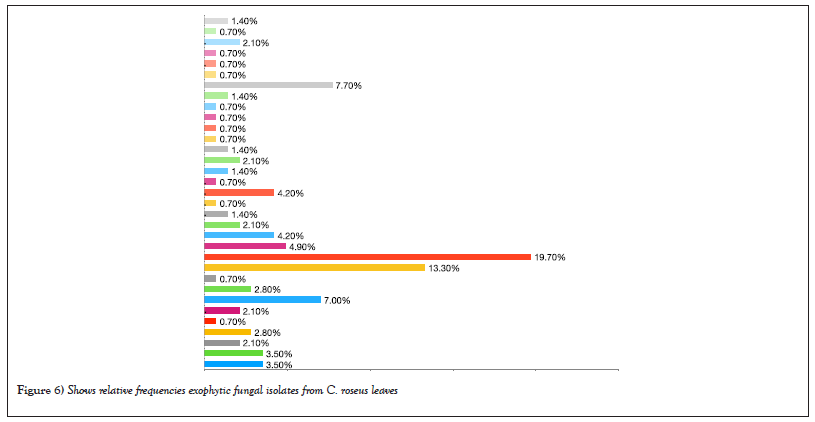
Figure 6: Shows relative frequencies exophytic fungal isolates from C. roseus leaves.
Medicinal plants have always been a niche for endophytes, and the host-microbial interaction has always instigated the production of several novel metabolites by microbes. The microbial diversity of the plant harbors and the chemo diversity that the fungi possess, provides ample opportunity to discover new compounds. Apart from the microbial and chemical diversity, the endophyte-host plant interaction also confers the potentiality to the microbes to produce a myriad of novel medicinally valuable compounds [29].
This study showed that the number of exophytic fungal isolates was higher than endophytic fungal isolates. This difference may be because although different plant species receive the same fungal propagules on the leaf surface, endophytic fungi are filtered more by host plants than exophytic fungi [30]. Plant species have various foliar physical characteristics and nutrient contents that can affect endophytic fungal colonization. For example, endophytic fungi need to penetrate host surfaces and absorb nutrients from hosts [31,32]. By contrast, exophytic fungi depend on nutrients deposited on leaves from the atmosphere or those exuded from leaves and are likely to be more affected by external environmental factors, such as wind speed, temperature, relative humidity, and solar radiation, rather than by hosts [33].
Colonization and relative frequency data show that different endophytic and exophytic fungi inhabited leave tissues differently. This difference inhabits the potential of the fungi to shows that each fungus exhibited different degrees of affinity towards different plant leaves. In our study, Aspergillus sp., P. chrysogenum, Cladosporium sp., and Chaetomium sp. were isolated as endophytes from the C. roseus plant in agreement with results published by Kharwar et al., [34], Momsia and Momsia [35], Dhayanithy et al., [15], Linh et al., [36]. According to our result, A. caespitosus were simultaneously isolated as endophytic and exophytic fungi, which agrees with Khan et al., [37]. They isolated A. caespitosus as endophyte from the Moringa peregrina plant. Piska et al., [28] isolated Arthrinium sp. and Cladosporium sp. as endophytes from a medicinal plant, which is supporting our result. Different exophytic species such as A. niger, A. flavus, P. commune and Cladosporium sp., were recently isolated as an endophytic from the medicinal plant [38,39]. While Kharwar found Nigrospora sp., Drechslera sp., Aspergillus sp., Cladosporium sp., Alternaria sp., and Penicillium sp. as exophytic isolated from leaf surfaces [40].
In addition, the result showed that dark-colored conidia such as Alternaria, Curvularia, Cladosporium and Nigrospora were more prevalent than dull-colored conidia Aspergillus and Penicillium. During germination, they are more tolerant of environmental factors, such as drought and high relative humidity. Exposure of Alternaria conidia's germination tube to damage during dehydration leads producing of another germination tube from a second cell of the multicellular conidia [41,42].
In conclusion, a huge variety of fungal communities may inhabit in medicinal plants. This work aimed compares the exophytic and endophytic fungal communities in C. roseus leaves. The results show that C. roseus leaves can be colonized by a wide range of exophytic or endophytic fungus species. These fungi undoubtedly impact the health and vitality of plants. Some endophytic may be exophytic and cause infection. At the same time, other’s may be unharmful to the plant. Certain species of endophytes produce different secondary metabolites include mycotoxins which affects plant, animal, and human. The discovery of new fungal species can provide new perspectives and opportunities for compounds and mycotoxins with medicinal value.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Alsubaie S, Bokhari F, Najjar A. Diversity of endophytic and exophytic fungi isolated from Catharanthus roseus L. G. Don. leaves in Saudi Arabia. AGBIR.2023;39(3):572-577.
Received: 12-May-2023, Manuscript No. AGBIR-23-98582; , Pre QC No. AGBIR-23-98582 (PQ); Editor assigned: 15-May-2023, Pre QC No. AGBIR-23-98582 (PQ); Reviewed: 29-May-2023, QC No. AGBIR-23-98582; Revised: 05-Jun-2023, Manuscript No. AGBIR-23-98582 (R); Published: 12-Jun-2023, DOI: 10.35248/0970-1907.23.39.572-577
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
