Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2024) Volume 40, Issue 4
Background: In developing countries food borne infection leads to death of many children and the resulting diarrheal disease can have a long term effect on children’s growth as well as on their physical and cognitive development. Meanwhile, food contamination from raw meat is an important cause of food borne disease outbreaks or food poisoning due to improper food handling.
Objective: The study aimed to assess bacteriological quality, antibiotic susceptibility pattern of bacterial isolation of raw meat, and associated factors among butcher houses of Adama town, Ethiopia, 2020.
Method: A cross sectional study design was conducted among 112 butcher shops in Adama town from October 1 to December 30, 2019. A simple random sampling method was used. Hereafter, 100 grams of raw meat was collected and transported to a referral laboratory center within 2 hr in an icebox for bacteriological analysis. Nutrient agar, Macckoncey agar and Mannitol salt agar were used to enumerate total aerobic plate count, Total coliform/fecal count and total Staphylococcus aureas count respectively with all media were from hardy diagnostic, America. The Kirby Bauer disk diffusion method was used to check susceptibility patterns of the potential pathogenic bacterial isolates. Data was entered into Epi-Info version 7.2 and analyzed usin g SPSSS oftware version 21.
Result: Three-fourth (¾th) of collected raw meat was an unacceptable bacterial load of total aerobic plate count based on gulf standard. The average contamination was (5.89 ± 0.86) log colony forming unit per gram for total aerobic plate count. Raw meat collected from meat handlers who trained on meat hygiene (Adjusted odd ratio=5.8,95% CI:1.99-17.34) and collecting money (Adjusted odd ratio 0.14,95% CI:0.04-0.43) were associated with the bacteriological quality of raw meat. Whereas, the proportion of meat samples that were positive for Salmonella and Shigella were (9.8% and 2.67%) respectively. The resistance of Salmonella was most frequently observed to Ampicillin (100%), Amoxicillin/Clavunilic (54.5%), Tetracycline (36.3%) Trimethoprim-sulfamethoxazole (18.2%). Shigella expressed resistance to Ampicillin (50%) and 100% sensitivity to the rest antibiotics used.
Conclusion and recommendations: The bacterial logarithmic mean values from the samples tested were beyond the acceptable standard and an indication of poor hygiene, making it a potential source of food borne infection. Therefore, stringent inspection, regular supervision, training, and hygienic practices should be introduced in order to enhance the overall hygienic quality of meat to safeguard consumers.
Aerobic plate count; Total coliform count; fecal coliform count; Staphylococci count; Amoxicillin
AHMC: Adama Hospital Medical College; APHRRLC: Adama Public Health Research and Referral Laboratory Center; ASP: Antimicrobial Susceptility Pattern; BPW: Buffered Peptone Water; CFU: Colony Forming Unit; EU: European Union; FAO: Food and Agricultural Organization; FBI: Food Borne Illness; HACCP: Hazard Analysis Critical Control Point; MSA: Manitol Salt Agar; NA: Nutrient Agar; NSS: Normal Saline Solution; OR: Odd Ratio; ORHB: Oromia Regional Health Bureau; PCA: Plate Count Agar; SPC: Standard Plate Count; TAPC: Total Aerobic Plate Count; TCC: Total Coliform Count; TSI: Triple Sugar Iron; WHO: World Health Organization
Raw meat refers to animal tissue used as food, mostly skeletal muscles, and associated fat but it may also refer to organs including lungs, livers, brains, bone marrow, kidneys and a variety of other internal organs as well as blood [1,2]. It is the major source of protein and valuable qualities of vitamin for most people in many parts of the world [3].
In developing countries, foodborne infection leads to the death of many children and the resulting diarrheal disease can have a long term effect on children’s growth as well as on their physical and cognitive development [4]. Meanwhile, food contamination from raw meat is an important cause of food borne disease outbreaks or food poisoning due to improper food handling [5].
Meat is rich in nutrients and highly susceptible to microbial contamination that can cause foodborne illness to consumers and meat spoilage. This can result in quality deterioration hence quantity losses, economic losses and public health concerns. Failure to observe good sanitation and hygiene practices such as the washing of hands, wearing of protective clothing, cleaning and sanitization of butchery equipment and utensils, transportation of meat in clean containers, and storage of meat at appropriately low temperatures can lead to microbial contamination, meat quality deterioration and post-harvest meat losses [6].
Contaminated raw meat is one of the main sources of food borne illness and is a risk factor for the transmission of zoonotic infection [7,8]. It offers a highly favorable environment for the growth of pathogenic microorganisms [9]. For this reason, retail meat is frequently associated with foodborne illness if infective doses are reached at the time of consumption.
Ideally, meat should be considered wholesome when pathogens of concern are absent or if present should be at a low number depending on their toxin or metabolites produced [10]. Bacteriological assessments of raw meat are used as an indicator of its hygienic quality [11]. The poor infrastructural facilities in slaughterhouses, unhygienic vending operations, and poor handling of carcasses attribute to the high bacterial load in meat [12].
Good sanitation and hygienic practices in butcher shops can reduce the level of both spoilage and pathogenic micro organisms in foods [13,14]. Microbial contamination of raw meat and meat products must not exceed levels that could adversely affect the shelf life and render it unwholesome and unfit for human consumption. Reducing meat contamination will reduce the risk of transmission of pathogenic bacteria and foodborne diseases to consumers.
Under tropical conditions, food of animal origin tends to deteriorate more rapidly and become an important vehicle for gastrointestinal infections, thereby endangering consumers' health [15]. Raw meat may harbor many important pathogenic microbes such as E. coli, S. aureus, Salmonella species, Campylobacter jejuni, Listeria monocytogenes etc., making such a meat a risk for human health [16,17]. The effective way of assessing meat quality is by using indicators of microbes (total aerobic plate count, total coliform count, and fecal coliform count).
The antimicrobial resistance of bacteria isolated from food and other sources has increased from time to time [18]. The main risk factor for the increase in antibiotic resistance is the extensive use of antibiotics in human health and animal [19]. There is evidence that patients infected with antibiotic resistance strains suffer more than those infected with sensitive strains [20].
Illness due to eating contaminated food is the most significant widespread health problem and an important cause of reduced economic productivity in the world. In the past, people in the world have worried about the role of meat and meat products in food poisoning but available records show that more than 74% of cases of food poisoning worldwide are due to meat dishes.
Raw meat consumption is a strong predictor of foodborne disease mortality. In a cross-country study for every additional metric ton of meat consumed per 100 people foodborne disease mortality increases by 6%. The widespread distribution of raw meat and traditional methods of handling, processing and marketing of meat undermine meat quality.
The Center for Disease Control and prevention (CDC) estimates that 48 million cases of food borne illness occur in the United states every year many of them caused by Salmonella and Eschersia species. It is reported that every year from 24 to 81 million cases of food borne illness are recorded in the USA, out of which 50% are associated with contaminated meat.
In most developing countries, the absence of non-respect for the existing hygienic practices in slaughtering, transportation and marketing has been found to be one of the major causes of meat contamination by pathogenic and non-pathogenic microorganisms.
It is generally recognized that the most significant foodborne hazards from raw meat are bacteria that can cause disease in humans (pathogenic bacteria), such as Salmonellae species, Staphylococcus aureus, Listeria monocytogenes, Campylobacter species, and Escherichia coli O157:H7. Some of these, particularly E. coli O157:H7, require only a few bacteria to cause food poisoning in humans.
Hygienic and quality control methods for meat and meat products, especially in food catering, have been recommended in many countries. But in Ethiopia, the widespread habit of raw meat consumption and lack of compliance with standard hygiene and sanitation protocols is a potential cause of food borne illnesses in the country. The presence of a meat inspection system examines grossly apparent abnormalities during the antemortem and post mortem examination but does not recognize complex microbial contamination, which could later precipitate major public health hazards.
Several scientists recommended the continuous investigation and inspection of raw meat to provide safe and wholesomeness for human consumption. The demand and consumption of animal products such as meat (especially raw meat) are high in Adama town but, reports on the hygienic status and handling practices of meat in butcher shops are very scarce. Thus, by assessing the bacteriological quality of raw meat threat posed to public health can be ascertained.
Drug resistant bacteria isolates is an emerging public health problem and the main risk factor for increased resistance is miss use of antibiotics for therapy and growth promoter in animal. In recent years, testing of bacteria has shown that an increasing proportion of isolates are resistant to several antimicrobial agents both in developing and developed countries.
Currently, 80% of all antibiotics are administered to livestock for better animal survival and higher meat yields. This high rate of antibiotic use can result in the development of antibiotic resistance in livestock associated bacterial species. Many bacteria that infect livestock also infect humans (e.g., E. coli, S. aureus, Salmonella).
There is growing scientific evidence that the use of antibiotics in food animals leads to the development of resistant pathogenic bacteria that can reach humans through the food chain. This underlines the need to limit the use of antimicrobials in veterinary practice to limit the occurrence of resistance.
Despite the high rate of consumption of raw meat, studies are lacking on the microbial evolution of raw meat quality in the study area hence the need for the present study. Therefore, investigating the bacteriological quality of raw meat, associated factors, and antimicrobial susceptibility pattern of isolates among butcher shops of Adama town will minimize public health risks associated with raw meat consumption in the study area, and in the country at large.
Hygienic evaluation of raw meat
The microbiological profile in food products is the key criteria for determining quality and safety. With regard to raw meat products, their safety and quality can be estimated based on microorganism counts, including TAPC, TCC and TFC. Their presence indicates the possibility of finding pathogenic bacteria. TAPC counts provide an estimation of the total microbial population and high levels of TAPC are usually correlated to low quality and reduced shelf life.
Total coliforms are a group of bacteria that are widespread in nature. All members of the group of the total coliforms can occur in human feces, but some can also be present in animal manure, soil, submerged wood, and in other places outside the human body. The usefulness of total coliforms as an indicator of fecal contamination depends on the extent to which the bacteria species found are fecal and human in origin.
Fecal coliforms are a good indicator of contamination from human or other animal waste products and they indicate a greater risk of exposure to pathogenic organisms than total coliforms. To prevent the occurrence of food borne illnesses and possible meat spoilage, it is important to ensure that the foods sold are safe, wholesome, and in good hygienic condition.
Total bacterial and coliform counts
A study on the bacteriological quality of raw meat conducted in the United Kingdom and Saudi Arabia revealed total aerobic counts were 6.11 log10 CFU/gm and 6 log10 CFU/gm in the UK and Saudi Arabia respectively. Another study from France on raw pork meat that the contaminations were log normally distributed with Enterobacteriaceae mean log counts ranging from 0.6 to 2.2 log10 CFU cm-2 and Pseudomonas log counts ranging from 1.1 to 4.4 log10 CFU cm-2.
A bacteriological survey of raw ground beef in San Francisco, California by using the most probable number method 96.7% of the 150 meat samples were positive for coliforms, 94.7% for Escherichia coli, and 61.3% for Staphylococcus aureus. Study on prevalence of Enterobacteriaceae in minced beef and beef burgers purchased from supermarkets and butcher shops in the Republic of Ireland. Overall, in the 43 beef products in which E. coli 0157:H7 was present and the Enterobacteriaceae counts ranged from 0.52 to 6.98 log10 CFU/gm.
In cross sectional study conducted in Chennai city, India mean Tota aerobic count, total coliform count and staphylococcal count on raw meat was 4.78 log10, 2.07 log10 and 5.16 log10 respectively CFU/gm. In a study on microbiological quality of minced meat samples marketed in Istanbul 60 meat samples were analyzed, number of TVC ranged between 2.7 × 104–2 × 108 cfu/g, while the numbers of total coliform bacteria, E. coli, S. aureus, Pseudomonas and molds/yeasts ranged between 3.5 ×102–4.5 × 107 cfu/g, 101–8.5 × 104 cfu/g, 6.5 × 102–3.7 × 106 cfu/g, 102–2.8 × 107 cfu/g and 7 × 103–4 × 108 cfu/g, respectively.
In a cross sectional study on 50 beef samples from a slaughter unit in Aizawland revealed an average bacterial count of 6.13 ± 0.09 log10 cfu/g and 12% positive for E. coli. In 68 raw meat samples collected in Iran, reported standard plate counts that varied from 103 to 2.6 × 106 cfu/g, Coliforms varied from <101 to 2.4 × 104 cfu/g, E. coli varied from <101 to 3 × 102 cfu/g, and S. aureus 5 × 102 cfu/g.
The study was conducted on S. aureus, E. coli, and Salmonella from raw meat at abattoirs and butcher shops in different areas of Lahore city, Pakistan 51% of samples had an Aerobic plate count of more than 6log10 CFU/gm, which indicates high contamination.
Other studies from Asia and eastern Nepal show 84%, 68% 34% Coliforms, S.aureu, and Salmonella species were isolated respectively from raw meat sold in retail shops. In a study conducted on raw meat Sold in Sylhet Sadar, in Bangladesh total aerobic count of the samples ranged between 2.5 × 105 to 2.25 × 105 cfu/g and 28% were rejected i.e. unacceptable for public consumption.
In the survey conducted on bacteriological quality and safety of raw beef from selected outlets in Windhoek, Namibia the overall prevalence of TPC on the beef samples was 95 (98.9%). Of these 95 (98.9%), 25 (26%) samples were satisfactory majority 47 (49%) samples were within acceptable level and 24 (25.0%) exceeded the acceptable level.
In a study to investigate the microbiological quality and safety of beef meat in East Java Province, Indonesia Most of the samples was contaminated with E. coli (32.5% positive samples) and S. aureus (20.0% positive samples). The mean values of TPC and S. aureus contamination were lower than the maximum limit of contamination, which was 41.58 CFU/g and 13.93 CFU/g, respectively, while the mean value of E. coli contamination was 27.03 CFU/g which was higher than the maximum limit.
In a study on the bacteriological quality of raw meat in Lafia metropolis, Nigeria show that the mean aerobic plate count, total coliform count, and total staphylococcal count were 1.94 × 107 cfu/g, 2.63 × 105 cfu/g respectively. In a similar study from North Africa, Morocco 23.8% of meat samples from butcher shops were above the recommended value set by WHO/FAO.
Another study from fresh meat retail shops in Bahir Dar, Ethiopia, reported mean Tota Aerobic count, total coliform count, and staphylococcal count were 4.53, 3.97 and 3.88 log10 cfu/g respectively. A similar study from Gonder town revealed meat quality from the butcheries exceeded the acceptable range of bacterial load (>5 log10 CFU/cm2) over the study period. In the study from Wolaita Sodo, Ethiopia Mean Aerobic plate count, total Coliform on meat samples collected were 5.91 × 106 cfu/g, 4.8810 6cfu/g respectively and Staphylococcus aureus was 20.3%. Study on the evaluation of the quality of beef produced and sold in parts of the Tigray Region of Ethiopia, a high percentage of samples (varying from 38.56%–to 84.34%) were of unsatisfactory quality.
Prevalence of bacterial pathogens in raw meat
A study done on raw beef in Washington D.C. area revealed 0.5% Campylobacter species, 19% Escherichia coli and 1.9% salmonellosis were identified. In a cross-sectional study done in the United Kingdom on 2330 raw meat of cattle Salmonella species and Campylobacter species were detected in 84 of 2330 (4%) and 15 of 2330 (0.6%) raw meat products, respectively.
In a study to investigate the bacteriological quality and safety of raw meat in Sweden E. coli O157, L. monocytogenes, and S. Typhimurium were isolated as meat borne pathogens. In a cross sectional study from 22 meat and meat products samples in Mumbai, India out of which 68.8% were coagulase positive S. aures. Another study from raw beef samples in Palestine The prevalence of S. aureus, Salmonella, and E. coli was 30%, 25% and 95%, respectively.
In 1636 processed food samples of meat, milk, and other food commodities from Pakistan confirmed the highest prevalence of Campylobacter in raw chicken meat (48%) followed by raw beef (10.9%) and raw mutton (5.1%).
Another study in Alexandar, Egypt conducted on the incidence of Enterobacteriaceae to determine the sanitary quality of raw meat products of cattle Escherichia coli (20%), Salmonella species (8%), Klebsiella species (6%), and Proteus species (10.6%) were isolated.
In keibe state Nigeria, a total of 49 isolates from raw meat including Bacillus subtilis 2 (4.1%), Proteus Vulgaris 3 (6.1%), Enterobacter species, 12 (24.5%), Pseudomonas aeruginosa 7 (14.3%), Escherichia coli 14 (28.6%), Salmonella species 3 (6.1%) and Staphylococcus aureus 8 (16.3%) were identified. In Addis Ababa, the overall prevalence of Salmonella isolated from minced meat beef, mutton and pork from retail supermarkets was 14.7%. Salmonella was detected in 14.4% minced beef, 14.1% mutton, and 16.4% pork samples subjected to isolation and identification.
In a cross sectional study on raw meat sold in butcher shops of Addis Ababa prevalence of L. monocytogenes was 5.5%, isolated. A similar study from Northern Ethiopia Gonder town, the prevalence of Shigella accounts for about 7.4% of raw meat sold at butcher shops (8,63). A study conducted in Dire-Dawa, Ethiopia shows that out of the 384 meat samples collected from the two abattoirs, a total of 61 (15.89%) meat samples were found to be positive for E. coli.
Factors affecting the bacteriological quality of raw meat in butcher shops
Bacterial contamination of raw meat originated from hairs, skin or hide of animals which are naturally contaminated by a variety of microorganisms and hence microbial contamination of carcasses normally occurs during skinning, evisceration, processing at abattoirs and retail outlets. Age and gender of the animal have a major influence on the quality of meat that is produced from animals.
The level of education and training of food handlers about the basic concept and requirements of personal hygiene and its environment plays an important part in safeguarding the safety of products to consumers. A study was done in Morogoro, Tanzania and Kenya butchers who receive training have good hygienic practices than their counterparts. Butchers in developing countries are untrained and thus, they pay no attention to the hygienic standards and as a result, contribute immensely to bacterial contamination.
As many as 109 counts of pathogenic microorganisms are present in the fingernails of people handling food due to poor personal hygiene practices such as negligence to wash hands after visiting the toilet. A study conducted in Kenya, the low usage of protective clothing in the butchery shops is indicative of an increased risk of microbial contamination of meat by butchery workers.
The practice of wearing protective clothes helps to reduce the burden of contaminants in meat. Regarding this, the Ethiopian ministry of agriculture recommends that personal clothing can carry microorganisms (germs) that have been gathered from a wide variety of sources into the meat or meat handling facility. Therefore, to protect meat and meat handling facilities from contamination because of personal clothing, protective overalls or hair covers should be worn at all times when handling meat. The wearing of jewelry, watches, and other detachable items should be discouraged. Dirt and organisms such as S. aureus can build up and around such items, and they pose a risk of foreign body contamination if they fall into the meat.
In addition to their clothes, the workers by themselves can be a probable source of contamination due to illness. It was recommended that new applicants could be examined clinically and bacteriologically before they are employed and at regular intervals afterward. The examination should include medical history to determine past infections with special reference to dysentery, typhoid, paratyphoid fevers, venereal and skin diseases and bacteriological examination of stool and urine.
During the transport of meat unprotected or poorly wrapped may be exposed to microbiological agents from the environment. Vehicles for transporting meat should be considered as an extension of the refrigerated storage. Uninsulated vans and open trucks are not suitable to transport for meat, particularly in hot climates. This is because in open trucks the meat is exposed to dust and attack from insects.
Antibiotic susceptibility pattern of isolated bacteria
In a cross sectional study on the prevalence, antibiotic susceptibility profiles and genotypes of S. aureus in the United States of America. Resistance (intermediate and complete) to tetracycline, ampicillin, penicillin and erythromycin was highly prevalent. The study conducted in Mexico on the antibiotic susceptibility pattern of Salmonella results showed that most antimicrobials tested were resistant except for cefotaxime, gentamicin, and kanamycin.
In the study done in India on raw beef meat, among the 15 isolates of E. coli tested for resistance against various antibiotics all the isolates (100%) were found to be resistant to erythromycin and streptomycin, followed by sulphadiazine (95.84%) and cephaloridine (87.50%). Moderately high resistance was detected towards cephalexin (41.69%), penicillin G (37.60%), ceftiofur (33.36%), norfloxacin (33.36%), enrofloxacin (27.40%) and carbenicillin (25.30%).
A meta-analysis study done in Ethiopia shows about 25% (95% CI: 10.0, 40.0) of the Salmonella species were found resistant to ampicillin. Besides, 9% (95% CI: 2.0, 15.0) of Salmonella species and 2% (95% CI: 0.0, 5.0) of E. coli O157:H7 isolates were found to be resistant to ceftriaxone. The pooled estimate indicated that 10% of E. coli O157:H7 isolates were resistant to ciprofloxacin. Salmonella species (6%), L. monocytogenes (5%), and E. coli O157:H7 (2%) were resistant to gentamicin.
In a study done in Adis Ababa, Gullele sub city E. coli isolates were observed to be the most resistant to penicillin (60%) followed by Amoxicillin (40%) and Ampicillin (40%) and none of the isolates were resistant to chloramphenicol. All isolates of Salmonella (100%) were resistant to penicillin and Vancomycin and 66.67% of the isolates were resistant to Ampicillin. None of the isolates were resistant to Ciprofloxacin. S. aureus isolates were resistant to penicillin (60%), Amoxicillin (40%) and Ampicillin (40%), and none of the isolates were resistant to Ciprofloxacin.
A study conducted in Jimma, Ethiopia revealed Shigella was susceptible to most of the antibiotics but resistant to Co-trimethoxazole, Tetracycline, Streptomycin and Trimethoprim. In the case of Staphylococcus aureus, 90% were resistant to Oxacillin, 85% to Ampicillin, 65% to Erythromycin, 60% to Amoxicillin, 35% to Streptomycin, and 20% to Vancomycin but all (100%) of the isolates were sensitive to Cotrimoxazole (90%). Staphyloccocus aureus isolates were Methicillin resistant In the case of Salmonella it was only resistant to Cephalexin.
The microbiological profile in food products is the key criteria for determining quality and safety with regard to raw meat products, their safety and quality can be estimated based on microorganism counts, including TAPC, TCC and TFC. Their presences indicate the possibility of finding pathogenic bacteria. TAPC counts provide an estimation of the total microbial population, and high levels of TAPC are usually correlated to low quality and reduced shelf life.
Total coliforms are a group of bacteria that are widespread in nature. All members of the total coliforms group can occur in human faeces, but some can also be present in animal manure, soil and sub merged wood and in other places outside the human body. The usefulness of total coliforms as an indicator of faecal contamination depends on the extent to which the bacteria species found are faecal and human in origin.
Faecal coliforms are good indicator of contamination from human or other animal waste products and they indicate greater risk of exposure to pathogenic organisms than total coliforms. To prevent the occurrence of food borne illnesses and possible meat spoilage, it is important to ensure that foods sold are safe, wholesome and in good hygienic condition.
Total bacterial and coliform counts
Study on bacteriological quality of raw meat conducted in United kingdom and Saudi Arabia revealed total aerobic count were 6.11log 10 CFU/gm and 6log 10 CFU/gm in UK and Saudi Arabia respectively. Another study from France on raw pork meat that the contaminations were log normally distributed with Enterobacteriaceae mean log counts ranging from 0.6 to 2.2 log10 cfu cm-2 and Pseudomonas log counts ranging from 1.1 to 4.4 log10 cfu cm-2.
A bacteriological survey of raw ground beef in San Francisco, California by using the most probable number method 96.7% of the 150 meat sample were positive for coliforms, 94.7% for Escherichia coli and 61.3% for Staphylococcus aureus. Study on prevalence of Enterobacteriaceae in minced beef and beef burgers purchased from supermarkets and butchers shops in the republic of Ireland. Overall, in the 43 beef products in which E. coli 0157:H7 was present and the Enterobacteriaceae counts ranged from 0.52 to 6.98 log10 cfu/gm.
In cross sectional study conducted in Chennai city, India mean total aerobic count, total coliform count and staphylococcal count on raw meat was 4.78log10, 2.07log10 and 5.16 log10 respectively CFU/gm. In a study on microbiological quality of minced meat samples marketed in Istanbul 60 meat samples were analyzed, number of TVC ranged between 2.7 × 104–2 × 108 cfu/g, while the numbers of total coliform bacteria, E. coli, S. aureus, Pseudomonas and molds/yeasts ranged between 3.5 × 102–4.5 × 107 cfu/ g, 101–8.5 × 104 cfu/g, 6.5 × 102–3.7 × 106 cfu/g, 102–2.8 × 107 cfu/g and 7 × 103–4 × 108 cfu/g, respectively.
In cross sectional study on 50 beef samples from slaughter unit in Aizawland and revealed the average bacterial count of 6.13 ± 0.09 log10cfu/g and 12% positive for E. coli. In 68 raw meat samples collected in Iran, reported standard plate counts that varied from 103 to 2.6 × 106 cfu/g, Coliforms varied from <101 to 2.4 × 104 cfu/g, E. coli varied from <101 to 3 × 102 cfu/g and S. aureus 5 × 102 cfu/g.
Study conducted on S. aureus, E. coli and Salmonella from raw meat at abattoirs and butcher shops in different areas of the Lahore city, in Pakistan 51% of samples had Aerobic plate count more than 6log10CFU/gm, which indicates high contamination.
Another studies from Asia, eastern Nepal shows 84%, 68% 34% Coliforms, S. aureus and Salmonella species were isolated respectively from raw meat sold in retail shops. A study conducted on raw meat sold in Sylhet Sadar, in Bangladesh total aerobic count of the samples ranged between 2.5 × 105 to 2.25 × 105 cfu/g and 28% were rejected i.e. unacceptable for public consumption.
Survey conducted on bacteriological quality and safety of raw beef from selected outlets in Windhoek, Namibia the overall prevalence of TPC on the beef samples was 95 (98.9%). Of these 95 (98.9%), 25 (26%) samples were satisfactory majority 47 (49%) samples were within acceptable level and 24 (25.0%) exceeded the acceptable level.
In a study to investigate the microbiological quality and safety of beef meat from in east java province, Indonesia most of the samples were contaminated with E. coli (32.5% positive samples) and S. aureus (20.0% positive samples). The mean values of TPC and S. aureus contamination were lower than the maximum limit of contamination, which were 41.58 CFU/g and 13.93 CFU/g, respectively, while the mean value of E. coli contamination was 27.03 CFU/g which was higher than the maximum limit.
In study on bacteriological quality of raw meat in Lafia metropolis, Nigeria show that mean aerobic plate count, total coliform count and total staphylococcal count were 1.94 × 107 cfu/g, 2.63 × 105 cfu/g respectively. Similar study from North Africa, morocco 23.8% of meat sample from butcher shops were above the recommended value set by WHO/FAO.
Another study from fresh meat retail shops in Bahir Dar, Ethiopia, reported mean total aerobic count, total coliform count and staphylococcal count were 4.53, 3.97 and 3.88 log10 cfu/g respectively. Similar study from Gonder town, revealed meat quality from the butcheries exceeded the acceptable range of bacterial load (>5 log10 CFU/cm2) over the study period. Study from Wolaita Sodo, Ethiopia mean aerobic plate count, total coliform on meat sample collected were 5.91 × 106 cfu/g, 4.88106 cfu/g respectively and Staphylococcus aureus was 20.3%. Study on evaluation of quality of beef produced and sold in parts of Tigray region of Ethiopia, a high percentage of samples (varying from 38.56%–84.34%) were of unsatisfactory quality.
Prevalence of bacterial pathogens in raw meat
Study done on raw beef in Washington D.C. area revealed 0.5% Campylobacter species, 19% Escherichia coli and 1.9% salmonellosis were identified. In cross sectional study done in United Kingdom on 2330 raw meat of cattle Salmonella species and Campylobacter species were detected in 84 of 2330 (4%) and 15 of 2330 (0.6%) raw meat products, respectively.
In a study to investigate the bacteriological quality and safety of raw meat in Sweden E. coli O157, L. monocytogenes and S. Typhimurium were isolated as meat borne pathogens. In cross sectional study from 22 meat and meat products samples in Mumbai, India out of which 68.8% were coagulase positive S. aures. Another study from raw beef samples in Palestine The prevalence of S. aureus, Salmonella and E. coli was 30%, 25% and 95%, respectively.
In 1636 processed food samples of meat, milk and other food commodities from Pakistan and confirmed highest prevalence of Campylobacter in raw chicken meat (48%) followed by raw beef (10.9%) and raw mutton (5.1%).
Another study in Alexandar, Egypt conducted on Incidence of Enterobacteriaceae to determine sanitary quality of raw meat product of cattle Escherichia coli (20%), Salmonella species (8%), Klebsiella species (6%) and Proteus species (10.6%) were isolated.
In Keibe state Nigeria a total of 49 isolates from raw meat including Bacillus subtilis 2 (4.1%), Proteus vulgaris 3 (6.1%), Enterobacter species, 12 (24.5%), Pseudomonas aeruginosa 7 (14.3%), Escherichia coli 14 (28.6%), Salmonella species 3(6.1%) and Staphylococcus aureus 8 (16.3%) were identified. In Addis Ababa, the overall prevalence of Salmonella isolated from minced meat beef, mutton and pork from retail supermarkets were 14.7%. Salmonella was detected in 14.4% minced beef, 14.1% mutton and 16.4% pork samples subjected to isolation and identification.
In cross sectional study on raw meat sold in butcher shops of Addis Ababa prevalence of L. monocytogenes was 5.5%, isolated. Similar study from Northern Ethiopia Gonder town, prevalence of Shigella accounts about 7.4% from raw meat sold at butcher Shops. A study conducted in Diredawa, Ethiopia shows that out of the 384 meat samples collected from the two abattoirs, a total of 61 (15.89%) meat samples were found to be positive for E. coli.
Factors affecting bacteriological quality of raw meat in butcher shops
Bacterial contamination of raw meat originated from hairs, skin or hide of animals which are naturally contaminated by a variety of microorganisms and hence microbial contamination of carcasses normally occur during skinning, evisceration, processing at abattoirs and retail outlets. Age and gender of the animal has a major influence on the quality of meat that is produced from animals.
The level of education and training of food handlers about the basic concept and requirements of personal hygiene and its environment plays an important part in safeguarding the safety of products to consumers. Study done in Morogoro, Tanzania and Kenya butchers who receive training have good hygienic practice than those counter parts. Butchers in developing countries are untrained and thus, they pay no attention to the hygienic standards and as a result contribute immensely to bacterial contamination.
As much as 109 counts of pathogenic microorganisms are present in the fingernails of people handling food due to poor personal hygiene practices such as negligence to wash hands after visiting the toilet. A study conducted in Kenya, the low usage of protective clothing in the butchery shops is indicative of increased risk of microbial contamination of meat by butchery workers.
The practice of wearing protective clothes helps to reduce the burden of contaminants in meat. Regarding this, the Ethiopian Ministry of agriculture recommends that personal clothing can carry microorganisms (germs) that have been gathered from a wide variety of sources into the meat or meat handling facility. Therefore, to protect meat and meat handling facilities from contamination because of personal clothing, protective over alls or hair cover should be worn at all times when handling meat. The wearing of jewelry, watches, and other detachable items should be discouraged. Dirt and organisms such as S. aureus can build up and around such items, and they pose a risk of foreign body contamination if they fall into the meat.
In addition to their clothes, the workers by themselves can be a probable source of contamination due to illness. It was recommended that new applicants could be examined clinically and bacteriologically before they are employed and at regular intervals afterwards. The examination should include medical history to determine past infections with special reference to dysentery, typhoid and paratyphoid fevers, venereal and skin diseases, and bacteriological examination of stool and urine.
During transport of meat unprotected or poorly wrapped may be exposed to microbiological agents from the environment. Vehicles for transporting meat should be considered as an extension of the refrigerated storage. Insulated vans and open trucks are not suitable transport for meat particularly in hot climates. This is because in open trucks the meat is exposed to dust and attack from insects.
The overall Aerobic Plate Count (APC) in raw meat
Antibiotic susceptibility pattern of isolated bacteria: In cross sectional study on the prevalence, antibiotic susceptibility profiles and genotypes of S. aureus in the United States of America. Resistance (intermediate and complete) to tetracycline, ampicillin, penicillin and erythromycin was highly prevalent. Study conducted in Mexico on antibiotic susceptibility pattern of Salmonella results showed that most antimicrobials tested was resistant except for cefotaxime, gentamicin and kanamycin.
The study done in India on raw beef meat, among the 15 isolates of E. coli tested for resistance against various antibiotics all the isolates (100%) were found to be resistant to erythromycin and streptomycin, followed by sulphadiazine (95.84%) and cephaloridine (87.50%). Moderately high resistance was detected towards cephalexin (41.69%), penicillin G (37.60%), ceftiofur (33.36%) and norfloxacin (33.36%), enrofloxacin (27.40%) and carbenicillin (25.30%).
Meta-analysis study done in Ethiopia shows about 25% (95% CI: 10.0, 40.0) of the Salmonella species were found resistant to ampicillin. Besides, 9% (95% CI: 2.0, 15.0) of Salmonella species and 2% (95% CI: 0.0, 5.0) of E. coli O157:H7 isolates were found to be resistant to ceftriaxone. The pooled estimate indicated that 10% of E. coli O157:H7 isolates were resistant to ciprofloxacin. Salmonella species (6%), L. monocytogenes (5%) and E. coli O157:H7 (2%) were resistant to gentamicin.
Study done in Adis Ababa, Gullele subcity E. coli isolates were observed to be the most resistant to penicillin (60%) followed by Amoxicillin (40%) and Ampicillin (40%) and none of the isolates were resistance for chloramphenicol. All isolates of Salmonella (100%) were resistant to penicillin and Vancomycin. And 66.67% of the isolates were resistance to Ampicillin. None of the isolates were resistance to Ciprofloxacin. S. aureus isolates were resistant to penicillin (60%), Amoxicillin (40%) and Ampicillin (40%) and none of the isolates were resistant to Ciprofloxacin.
Study conducted in Jimma, Ethiopia revealed Shigella was susceptible for most of antibiotics but resistant for Co-trimethoxazole, Tetracycline, Streptomycin and Trimethophrim. In case of Staphylococcus aureus 90% were resistant to Oxacillin, 85% to Ampicillin, 65% to Erythromycin, 60% to Amoxicillin, 35% to streptomycin, and 20% to Vancomycin but all (100%) of the isolates were sensitive to Cotrimoxazole (90%). Staphyloccocus aureus isolates were methicillin resistant in case of Salmonella it was only resistant to Cephalexin.
Conceptual framework of the study
For the construction of the following conceptual frame work, factors associated with poor quality of raw meat adopted from different studies used (Figure 1).
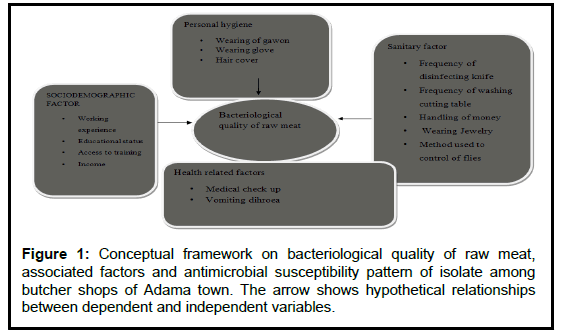
Figure 1: Conceptual framework on bacteriological quality of raw meat, associated factors and antimicrobial susceptibility pattern of isolate among butcher shops of Adama town. The arrow show
The study designed to assess bacteriological quality, its associated factors and antibiotic susceptibility pattern of the isolated raw meat from butcher shops of Adama town from October 1 to December 2019, Oromia Ethiopia. Study design was Cross sectional study design conducted from October 1 to December 30, 2019. Source population was all the butcher shops in Adama town. Study population was all butcher shops in which cattle meat were sold. Study unit was butcher shops in which sample were actually collected. Inclusion criteria was Butcher shops which used to sell meat of cattle originally and exclusion criteria was Butcher shops in which goat’s and sheep’s meat sold; Butcher shops which closed and shifted their task during the study period and non-volunteer (Table 1).
| Microbial groups (CFU/g) | Acceptable | Borderline | Unacceptable | Potentially hazardous |
|---|---|---|---|---|
| Total aerobic plate count | <4log | 4log__5log | >5log | NA |
| Total coliform count | <2log | 2log__4log | ≥ 4log | NA |
| Total fecal coliform count | <2log | 2log__3log | ≥ 13log | NA |
| S. aureus count | <2log | 2log__3log | 3log_4log | ≥ 4log |
| Pathogens | _____ | _____ | Detected in 25 gram |
TABLE 1: Guideline levels for determining the microbial quality of ready to eat food (Gulf standards).
Sample size and sampling technique
All 119 butcher shops which were working during study period were included in the study and simple random sampling method was employed to select meat handlers for interview.
Data collection tools: A pre tested structured questionnaire initially developed in English and then translated in to local language translation expert and then translated back to English by another person to check its consistency. The questionnaire structured into three distinct parts including demographic information such as respondents’ gender, age, years of experience, medical checkup and attending meat safety training. The second section of the questionnaire is about meat safety knowledge. Questions on knowledge referred to mainly about their personal hygiene, cross contamination and temperature. It contains 15 close ended questions and each question has three optional answers (“yes”, “no” and “I do not know”). The response was analyzed as categorical variables (right or wrong answer). A score of one was given to right answer and zero to the wrong and I do not know answer. The last section dealt with meat hygiene practices. The question comprises the issues of personal hygiene, hand washing practices, practices against food borne diseases and cross contamination. This section had 17 questions with two possible responses: “yes”, and “no”. Each correct practice reported scored one point.
Observational check list
Observational check list was developed after reviewing relevant literatures to assess the butcher shops hygienic status and practice. The check list incorporated personal hygiene of meat handlers and hygienic conditions of the butcher shops premises.
Data collection procedure: Face to face interview and the general sanitary condition of the butcher shops as well as the workers were observed. After finishing of questionnaires one hundred gram of raw meat sample was collected for laboratory investigation.
Data quality assurance: The data collectors were selected based on their educational background (two environmental health’s) and the selected data collectors were trained on the purpose and objective; benefit of the study, individual’s right, informed consent and techniques of the interview for one day. Daily checks up of data completeness were made by the principal investigator. The questionnaire was pre tested on 5% of butcher shops in Olanciti town neighboring town 25 km to Adama town before the study. The structured questionnaire was then rephrased in the light of the responses.
Statistical analysis: Before analysis, data were checked for completeness, consistencies and entered into computer using Epi info version 7.2.3.1software. Then the data was exported to SPSS version 25, coded, categorized, sorted and cleaned to facilitate analysis. Descriptive statistics was computed for the study variables and frequency distribution tables were used to describe most of the findings. All bacterial counts were normalized to CFU/g and converted into Log10 values. Mean and standard deviation were also computed. Variables with p-value less than 0.25 in binary logistic regression analysis were entered to binary multiple logistic regression using enter methods to determine factors independently associated with bacteriological quality of raw meat. Odds ratio with their 95% confidence intervals were computed to identify the presence of association and statistical significance were declared if p value is <0.05.All other assumptions of the analysis like normality of variables were checked. Odd ratio was considered to assess the strength of association between dependent and independent variables.
Laboratory work
One hundred gram of pooled raw meat cuts from leg area, limb area and flank area of hanging display for sale were collected from butcher shops in a sterile zipped plastic bag in an icebox. The samples were collected in the morning (9:00 am-10:00 am), after labelling properly; they were kept in an ice box between 2°C-4°C and were immediately transported to Adama public health research and referral laboratory center in Adama town Oromia, Ethiopia. The samples were analysed immediately upon arrival in the laboratory. From 100 g of grinded and homogenized meat 25 g was weighted and placed in 225 ml sterile 0.1% buffered peptone water. The grinded meat and diluent were thoroughly vortexed on a platform shaker for 5 minutes to wash off and dislodge any microbe that may be resident on the surface of the meat. The mixture was considered to be a 10-1 dilution. The mixture (1 ml) were transferred to a tube containing 9 ml of normal saline diluent to make 10-2 dilution. Further dilutions were made by transferring 1 ml of the succeeding dilutions to the tubes containing 9 ml diluent up to 10-6. After preparation, bacteriological analyses of the samples were performed to assess the selected microbial attributes such as Total aerobic plate count, Total coliform count, fecal coliform count and Total Staphylococcus aureus Count (TSC) in cattle meat by using Plate Count (PC) agar, Mac Conkey (MC) agar and manitol salt agar. All the media used were from hardy diagnostic, America.
Determining total aerobic plate count and judging meat quality
For the enumeration of total aerobic bacteria in raw meat samples conventional standard plate count method was used. Tenfold serial dilution up to 10-6 was made from the homogenized sample. One mL from each serial dilutions (10-3,10-4 ,10-5 and10-6) of the test sample was pipetted into sterile Petri dishes and then molten, cooled nutrient agar was added and incubated for 24 h at 37°C. Plates with colonies lying between 30-300 were counted using colony counter (TT20, Techmel, USA) and the average count was calculated and expressed as log CFU/gm. After determining TAPC by counting each visible colony of bacteria, the quality of each raw meat samples were judged based on Guideline levels for determining microbial quality of ready to eat food (Gulf standards). Meat samples of TAPC <5log 10 CFU/gm were acceptable and >5log 10 CFU/gm were unacceptable.
Enumeration of total coliforms and fecal coliforms
For the TCC and FCC 0.1 ml of each of dilution from 10-1, 10-2 and 10-3 was transferred and spread on triplicate on Mac Conkey (MC) agar. Then plates were incubated at 37°C and 44°C for 24 hours for TCC and for FCC counts respectively. Enumeration of the TCC and FC (typical pink colonies resulting from the fermentation of lactose). For Staphylococci aures count, mannitol salt agar (MSA, hardy diagnostic) was surface plated with 0.1 ml of the homogenate from duplicates of 10-1 and 10-2. The inoculum was evenly spread on the surface of the agar and allowed to dry for 15 min at room temperature. The plates were inverted and incubated for 24 to 48 h at 37°C. Typical colonies of Staphylococci aures (golden yellow colonies shining and convex) after 24 hours incubation were isolated, purified and tested for catalase and coagulase positive as a confirmatory test.
Isolation of Salmonella and Shigella
For the isolation of Salmonella and Shigella samples were pre enriched in buffered peptone water (incubated aerobically at 37°C for 24), followed by secondary enrichment in selenite cysteine broth (incubated aerobically at 37°C for 24) and plated on to XLD incubated aerobically at 37°C for 24, the suspected colonies were sub cultured on the blood agar and incubated at 37°C for 24 hr. Further identification was made with Triple Sugar Iron agar (TSI), urea broth, Lysine Iron Agar (LIA), citrate broth and then incubated for 24 to 48 hours at 37°C. All biochemical test reagents were obtained from hardy diagnostic, America. Antimicrobial susceptibility tests were performed using the modified Kirby-Bauer disk diffusion technique. Bacterial suspension turbidity was adjusted to 0.5 Mc Farland standard. A sterile swab stick was immersed into bacterial suspension and spread on surface of Muller-Hinton agar Commercially disks Amoxicillin/Clavunilic acid(20/10 μg), ceftriaxone (30 μg), erythromycin (15 μg), trimethoprimsulfamethoxazole (12.5/23.75 μg), tetracycline (30 μg), gentamycin (10 μg), ampicillin (10 μg) and ciprofloxacin (5 μg); all were from hardy diagnostic, America were used. Antimicrobial agents were selected based on clinical significance and literature data search. The results were interpreted using Clinical Laboratory Standards Institute (CLSI), 2019 guideline E. coli (ATCC 25922) was used as quality control organism for the antimicrobial susceptibility testing.
Culture media quality control
Quality of culture media was maintained after checking its expiration date and preparation according to manufacturer instruction by sterilizing at 121°C (15 lbs. sp) for 15 minutes. Sterility of culture media were also checked using strains kept for quality checking at APHRRLC. To exclude lab contaminants and check whether media and diluent completely sterilized, a representative number of a plate with media and broth without the test sample were incubated at 37°C for 48 hours. If any growth observed on control media, this batch will be discarded and another media will be replaced. Gram staining reagents were also checked for their expiry dates of each reagent, their storage condition and checked with known quality control organisms (ATCC, American type culture collection Organism) before performing study samples. S. aureus ATCC 25923, E.coli ATCC 25922, Shigella flexineri ATCC 12022 and Salmonella typhimurium ATCC 14028 were used as quality control reference strains.
A total of 112 study participants were involved, making a response rate of 112/119 (94%). The mean age of the participants was (32.83 ± 8.31) years (Table 2).
| Variables | Frequency | % | Mean ± SD | Range |
|---|---|---|---|---|
| Age | ||||
| <20 | 12 | 10.7 | ||
| 21-30 | 32 | 28.6 | 32.830 ± 8.3 | 17-54 |
| 31-40 | 47 | 42 | ||
| 41-50 | 20 | 17.9 | ||
| >51 | 1 | 0.9 | ||
| Marital status | ||||
| Married | 76 | 67.9 | ||
| Single | 30 | 26.8 | ||
| Others* | 6 | 5.4 | ||
| Religion | ||||
| Orthodox | 71 | 63.4 | ||
| Muslim | 22 | 19.6 | ||
| Protestant | 19 | 17 | ||
| Level of education | ||||
| Illiterate | 3 | 2.7 | ||
| Primary | 56 | 50 | ||
| Secondary | 37 | 33 | ||
| Diploma | 10 | 8.9 | ||
| Degree | 6 | 5.4 | ||
| Working experience | ||||
| <5 | 29 | 25.9 | 2-20 | |
| 5-10 | 32 | 28.6 | 9 ± 4.543 | |
| >10 | 51 | 45.5 | ||
| Meat safety training | ||||
| Yes | 45 | 40.2 | ||
| No | 67 | 59.8 | ||
| Medical check up | ||||
| Yes | 36 | 32.1 | ||
| No | 76 | 67.9 | ||
*divorced, widowed
TABLE 2: Socio demographic, Socioeconomic others and characteristics of study participants (n=112)
Observation of butcher shops
Sixty nine (61.6%) of butcher shops wall and ceiling made of ceramic. None of meat handlers weared hand glove. Eighty five point seven persent of meat handlers did not wear head cover. Moreover, sixty four (57%) of the butcher shops have no cashiers and they collect money while handling meat (Table 3).
| Variable | Frequency | Percentage (%) |
|---|---|---|
| Butcher shop wall and ceilings made of | ||
| Ceramic | 69 | 61.6 |
| Concrete | 31 | 27.7 |
| Others* | 12 | 10.7 |
| Butcher shop wall and ceilings free of dusts and spider web | ||
| Yes | 42 | 37.5 |
| No | 70 | 62.5 |
| Meat handlers wear white coat | ||
| Yes | 61 | 54.5 |
| No | 51 | 45.5 |
| Meat handlers wear head cover | ||
| Yes | 16 | 14.3 |
| No | 96 | 85.7 |
| Meat handlers wear glove | ||
| Yes | - | - |
| No | 112 | 100 |
| Handling money while selling meat | ||
| Yes | 64 | 57 |
| No | 48 | 43 |
| Wear Jewelers | ||
| Yes | 44 | 39.3 |
| No | 68 | 60.7 |
*Earthen materials, Aluminum
TABLE 3: Hygiene of butcher shops premises and meat handlers in Adama town, Oromia Ethiopia from October 1 to December 30/2019 n=112.
Meat handlers and meat hygiene knowledge
Overall knowledge level of respondents about personal hygiene, cross contamination and transmission of food borne diseases summarized in Tables 4 and 5.
| Statements on meat handling practices | Write no % | Wrong | Do not know | |
|---|---|---|---|---|
| 1 | Improper handling of meat could pose health hazards to consumers? | 112 (100) | 0 | 0 |
| 2 | Do you know insects and pests could be a source of contamination to meat? | 101 (90.2) | 8(7.1) | 3 (2.67) |
| 3 | Do you know regular washing of hands during meat processing reduces risk of meat contamination? | 109 (97.3) | 3 (2.67) | 0 |
| 4 | Do you know using gloves while handling meat reduces the risk of meat contamination? | 53 (47.3) | 42 (37.5) | 17 (15.2) |
| 5 | Do you know washing and disinfection of butchery utensils reduces the risk of meat contamination? | 110 (98) | 2 (2) | 0 |
| 6 | Do you know microbes be in the skin, nose and mouth of health people? | 64 (57) | 23 (20.5) | 25 (22.3) |
| 7 | Do you know people with open skin injury, gastroenteritis, and ear or throat diseases should not be allowed to handle meat? | 88 (78.6) | 24 (21.4) | |
| 8 | Do you know the health status of meat handlers should be checked before employment? | 62 (55.3) | 11 (9.8) | 39 (34.8) |
| 9 | Do you know meat handlers with wounds or injuries on their hands must not touch or handle meat? | 21 (18.8) | 28 (25) | 63 (56.2) |
| 10 | Do you know regular rotation of disinfectants for cleaning reduces the risk of meat contamination from working surfaces and cutting material? | 110 (98) | 2(2) | 0 |
| 11 | Do you know diarrheal disease can be transmitted by food? | 94 (83.9) | 6 (5.4) | 12 (10.7) |
| 12 | Do you know contaminated raw meats transmit food borne pathogens to humans? | 99 (88.3) | 2 (2) | 11(9.8) |
| 13 | Do you know high temperature or freezing is a safe method to destroy bacteria? | 108 (96.4) | 0 (0) | 4 (3.6) |
| 14 | Do you know eating and drinking in the work place increase the risk of meat contamination | 74 (66) | 20(17.9) | 18 (16.1) |
| 15 | Do you know cross contamination is when microorganisms from a contaminated meat are transferred by the meat handler’s hands or utensils to another? | 100 (89.3) | 7 ( 6.3) | 5 (4.5) |
| Total | 87.5 | 11.8 | 0.7 |
TABLE 4: Summary of meat handlers and meat safety knowledge .
| Meat safety practices questions | Responses no (%) | ||
|---|---|---|---|
| Yes | No | ||
| 1 | Do you wash your hands before and after handling meat? | 109 | 3 |
| 2 | Do you use gloves while handling meat? | 0 | 112 |
| 3 | Do you smoke inside meat processing areas? | 0 | 112 |
| 4 | Do you wash hands after handling waste/garbage? | 112 | 0 |
| 5 | Do you wash hands after using toilet? | 112 | 0 |
| 6 | Do you wear a gawon while working? | 72 | 40 |
| 7 | Do you wear hair cover while working? | 21 | 91 |
| 8 | Do you frequently clean the meat storage area before storing new products? | 88 | 24 |
| 9 | Do you use the sanitizer when washing service utensils (knives, hooks and cutting boards)? | 99 | 13 |
| 10 | Do you replace knives or sterilize them after meat processing? | 59 | 53 |
| 11 | Do you remove your gown when using toilets? | 108 | 4 |
| 12 | Do you remove your personal stuffs such as rings, watch while processing meat? | 74 | 38 |
| 13 | Do you handle/process meat while you are ill? | 55 | 57 |
| 14 | Do you collect money while handling meat? | 52 | 60 |
| 15 | Do you eat or drink at your work place? | 66 | 46 |
| 16 | Do you wash your hand after sneezing or coughing? | 60 | 52 |
| 17 | Do you process meat when you have cuts, wounds, injuries on your hands? | 58 | 54 |
TABLE 5: Summary of meat handlers and hygiene practices .
Bacteriological quality of meat
Raw meat samples collected form butcher shops during the study period 85/112 (75.89%) have unacceptable bacteriological quality based on gulf standard. The enumeration of the TAPC ranged between 3.70log 10 cfu/g to 7.43log 10 cfu/g with an average count of 5.89log 10 cfu/g. Enumeration of TCC ranged 2.73log 10 cfu/g-5.76log 1cfu/g with an average of 4.27lo1cfu/g, whereas FCC and TSAC had mean of 3.1cfu/g and 3.0cfu/g respectively (Table 6).
| Microbial indicators | Minimum count (log10 cfu/g | Maximum count (log10 cfu/g) | Mean ±SD | Gulf standards maxpermissible count (log10 cfu/g) |
|---|---|---|---|---|
| TAPC | 3.7 | 7.43 | 5.89 ± 0.864 | 5 log10 cfu/g |
| TCC | 2.77 | 6.67 | 4.27 ± 0.73 | 4 log10 cfu/g |
| FCC | 0 | 5.67 | 2.77 ± 1.37 | 3 log10 cfu/g |
| TSAC | 0 | 5.91 | 3.02 ± 1.54 | 3 log10 cfu/g |
TABLE 6 : Bacteria loads of raw meat collected from Adama town butcher shops, Ethiopia from October 1 to December 30/2019.
In bivariate analysis, training of meat handlers, practices such as wearing white coat, head cover, collecting money and washing using sanitizer were significantly associated (p-value less than 0.25) with overall bacteriological quality of raw meat and moved to multivariable logistic regression model.
However in multivariable logistic regression model training and collecting money while handling meat were significantly associated (p-value less than 0.05) with bacteriological quality of raw meat in the butcher shops (Table 7).
| Variables | Bacteriological quality of raw meat | COR (95%CI) | AOR (95%CI) | p-value | |
|---|---|---|---|---|---|
| Acceptable no (%) | Unacceptable no (%) | ||||
| Receive training | |||||
| Yes | 20 (17.85) | 25 (22.3) | 6.8 (2.56-18.34) | 5.8 (1.99-17.34) | 0.001* |
| No | 7 (6.25) | 60 (53.57) | 1 | 1 | |
| Wear white coat | |||||
| Yes | 18 (16%) | 43 (38.4) | 1.9 (0.78-4.88) | 1.3 (0.41-4.32) | 0.6 |
| No | 9 (8) | 42 (37.5) | 1 | 1 | |
| Wearing head cover | |||||
| Yes | 7 (6.25) | 9 (8) | 2.95 (0.98-8.91) | 2.2 (0.5-9.6) | 0.26 |
| No | 20 (17.85) | 76 (67.8) | 1 | 1 | |
| Collect money | |||||
| Yes | 6 (5.4) | 58 (51.78) | 0.13 (0.045-0.36) | 0.14 (0.04-0.43) | 0.01* |
| No | 21 (18.75) | 27 (24) | 1 | 1 | |
| Washing using sanitizer | |||||
| Yes | 26 (23.2) | 73 (65) | 4.21 (0.5-34) | 1.9 (0.21-17.7) | 0.54 |
| No | 1 (0.9) | 12 (10.7) | 1 | 1 | |
Note: *p-value<0.05, crude odds ratio adjusted odds ratio
TABLE 7: Factors associated with bacteriological quality of raw meat .
In this study, from a total of 112 samples, 11 (9.8%) of them were designated as positive for the presence of Salmonella species. Whereas only 3/112 (2.68%) sample was shown to be positive for presence of Shigella species (Figure 2 and Table 8).
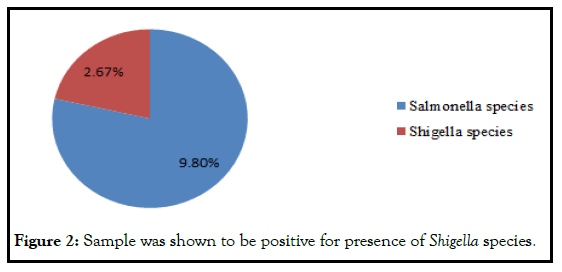
Figure 2: Sample was shown to be positive for presence of Shigella species.
| Bacterial isolates | Patterns | Antimicrobial agents | |||||||
|---|---|---|---|---|---|---|---|---|---|
| APX (10 µg) | AMX\C (20\10 µg) | SXT 12.5 µg\23.75 | TET (30 µg) | CPX (5 µg) | ERY (15 µg) | GEN (10 μg) | CFX (30 µg) | ||
| Salmonela | S | - | 5 (45.45%) | 11 (81.8%) | 7 (63.6%) | 11 (100%) | 11 (100% | 11 (100%) | 11 (100%) |
| I | 2 (18.2%) | - | - | - | - | - | - | - | |
| R | 9 (81.8%) | 6 (54.5%) | 2 (18.2%) | 4 (36.3%) | - | - | - | - | |
| Shigella | S | 1 (50%) | 2 (100%) | 2 (100%) | 2 (100%) | 2 (100%) | 2 (100%) | 2 (100%) | 2 (100%) |
| I | - | - | - | - | - | - | - | - | |
| R | 1 (50%) | - | - | - | - | - | - | - | |
APX: Ampicillin; AMX\C: Amoxicillin\Clavunilic; SXT: Trimethoprim; Sulfamethoxazole; TET: Tetracycline; CPX: Ciprofloxacin; ERY: Erythromycin; GEN: Gentamycin; cefx s: Sensitive; I: Intermediate; R: Resistant
TABLE 8: Antibiotic susceptibility pattern of bacterial isolates in raw meat .
Overall study participants (59.8%) (95% CI: 50,69) of meat handlers had not taken training on safe meat handling and personal hygiene. Similar with study done in Mekelle, Ethiopia, where 58.4% of meat handlers had not taken trainings related to personal hygiene and meat handling. Even though regular medical examination is recommended for food handlers by WHO, In this study seventy three (65.2.%) (95% CI: 57.1, 74.1) of meat handlers did not have evidence of medical certificate. This study confirms that although there exist personnel medical health requirements in Ethiopia there is very little attention given to their implementation and enforcement in a food enterprise like butcher shops. Therefore, there is a high possibility of the meat handlers contaminating meat with microorganisms. Handling of meat and money with the same unwashed hands is one sources of meat contamination. Results of this study revealed sixty four (57.1%) (95% CI: 48.2,66.1) of the meat handlers handled money (papers/coins) which may result into cross contamination of meat with microbes. Similar studies in Mekelle, Ethiopia 91.7% of the meat handlers collect money while serving meat. According to compliance study based on gulf standard raw meat in this classifies 85/112 (75.89%) (95% CI: 67.9,83.9) of meat have unacceptable bacteriological quality. Comparable findings were also obtained in meat retail shops of Meknes city, Morocco, reported a total aerobic plate count of 67% in beef produced and marketed with unacceptable quality. It is higher than in study done in Sylhet Sadar, Bangladesh in which 28% of meat were unacceptable quality. However, it is lower than study done in bahir in which all samples or hundred percent unacceptable. According to food and agricultural organization Total aerobic plate counts exceeding 5.0 log10 on fresh meat are not acceptable and alarm signals on meat hygiene.
The average TAPC was 5.89log CFU/g (95% CI: 5.7,6.1). Finding of this result is higher than East Java, Indonesia where mean of TAPC was 4.158 CFU/g and Chennai city, India (4.78log10). However it is less than Addis Ababa, Ethiopia (6.44 log CFU/g). The variations of bacterial load observed in different studies might be due to lack of good processing, handling practices, sampling and sanitary standard operating procedures of meat handlers. Raw meat collected from butchers who trained on meat safety hygiene was 5.8 times more likely to be acceptable than those who did not receive training (AOR=5.8,1.99-17.34). This is because training of food handlers about the basic concept and requirements of personal hygiene and its environment plays an important part in safeguarding the safety of products to consumers. Regarding collecting money, in the current study, raw meat which were collected from butcher SHOPS in which meat handlers handle money while selling meat was 86% less likely to be acceptable than their counter part (AOR=0.14,0.04-0.43). According to WHO/FAO report, handlings of foods with bare hands result in cross contamination and high microbial load. Furthermore, WHO recommends food handlers should be educated, encouraged or supervised to stop their business promptly if at any time, they suffer from diarrhea, vomiting, fever, sore throat or have visibly skin lesions. Even though it is not independent predictor in this study, fifty percent of meat handlers had practice of working while they were ill. With regard to contamination by total coliform, the average is 4.27 logCFU/g (95% CI: 4.1,4.4). This value is higher than that of commercial beef meat in Tanzania (4.13log CFU/g) and in India (2.07 log CFU/g) but lower than that found in Lafia metropolis, Nigeria (4.19). Variations in total coliform counts among studies may be due to differences in storage conditions and season in which samples were collected. The average contamination of meat by feacal coliform is 2.77log CFU/g, (95% CI: 5.7,6.1) it The result is lower than that of retail beef meat in Algeria (3.41 log CFU/g and higher than in beef meat of Namibi (1.70 log10 CFU/g).
The data in the present study indicate that 81 (72.3%) of samples collected in the town showed contamination with faecal coliforms. Presence of faecal coliforms suggests faecal contamination which is normally associated with poor hygiene and faulty slaughtering. It also suggests the possibility of finding enteric pathogens such as Salmonelll, Shigella and others. The average contamination of meat by Staphylococcus aureus is 3.14logCFU/g, (95% CI: 2.9,3.3). This value is higher than that of commercial beef meat in Chennai city, India (2.07log10). However, it is lower than study done in Bahir dar, Ethiopia. The highest number of S. aureus on meat indicates the presence of cross contamination, which usually related to human skin, hair, hand and discharge from nose, and clothing. Concerning the prevalence of pathogenic bacteria Salmonella was detected in 11 (9.8%) of analyzed samples. This finding reveled that there was a considerable rate of contamination in the butcher SHOPS of Adama town, which potentially poses a risk of causing food associated illness. The prevalence reported in the current study is higher than other reports such as in United State of America (6%). However, the result of this study was much lower than that found in Senegal (87%) and Bahir Dar (70%). This difference possibly arises from the source of animals, types of samples, and sampling technique. With regard to the antimicrobial susceptibility profiles of Salmonella isolates revealed a higher rate of resistance against Ampicillin 9 (81.8%) and Amoxicillin 6 (54.5%). These findings is in agreement with (86), where salmonella was 100% resistant to Ampicillin. An intermediate resistance of 2 (18.2%) was also found for Ampicillin. On the other hand, interestingly all of the isolates were 100% susceptible to gentamycine, ciprofloxacin, tetracycline and ceftraxione. This is in line with the study conducted in Ghana.In addition 7 (63.6%) and 9 (81.8%) exhibited susceptibility to Tetracycline and Trimethoprim-sulfamethoxazole respectively. This result is also in align with study done in Gondar, Ethiopia. However, resistance to Ampicillin is much higher than in Bahir dar (23.8%).
In other way the study revealed an overall Shigella prevalence of 2.67% which is higher than study done in Jimma, Ehiopia but, lower than in Karachi, Pakistan and Gondar, however, similarly lower rate of isolation was reported from Ebony, Nigeria. The antimicrobial susceptibility profiles of the isolates revealed resistance against ampicillin 1 (50%), but, all the isolates were sensitive to Amoxicillin, Tetracycline and Trimethoprim Sulfamethoxazole, Erythromycin gentamycin, Ciprofloxalin and ceftraxione. However, higher rate of resistance against ampicillin was observed in Gondar. Differences in the geographical location of the isolates or the emergency of drug resistant strains could partially explain this discrepancy.
Compliance study based on gulf standard 75.9% of raw meat collected from butcher shops has unacceptable bacteriological quality. Under observation more than half of meat handlers in the butcher shops handle money with their bare hands while processing of meat and serving of custom. The following areas are need big attention and concern for future suggestion to: Adama town ablators enterprise should get training on how to cope with good handling practices on the use of proper clothing such as hand gloves, head covers, clean white coat and dedicated cashier to collect money. Adama health bureau should regular be inspected butcher shops in the town. Owners of butcher shops should be dedicated cashier in order to collect money and consumers refrain from eating raw meat appropriately to avoid intoxication and infection due to microbes. Further investigation should be carried out to isolate and characterize the bacterial load of raw meat along with meat production chain.
Ethical clearance was obtained from Adama hospital medical college Institution Review Board (IRB). An official supporting letter was written by Adama Hospital Medical College to Oromia Regional Health Bureau for an ease of the study process and permission. Oromia Health Bureau was writing supporting letter to Adama Town Administration and then Adama Town Administration write supporting letter to selected Butcher shops in Adama town. The purpose and benefit of the study along with their right to refuse were explained to all butchers available during data collection period. For those potentially hazardous pathogens obtained during laboratory investigation, immediately re-inspect the butcher shops and take an action to solve the problem.
There is no identifiable details on individual participants reported in the manuscript, so, consent to publish is not required.
The datasets examined during this study is available from the both authors corresponding and others authors on sensible inquiry.
As the authors we did not have any competing interest.
We were proposed and competed study design, data collection, performed data analysis and recruited the manuscript. Third persons were critically revised and finalized entire document with important contributions. Finally, we all authors have read and approved the final version of the manuscript.
Aschalew Abebe (BSC in lab technology) working at Oromia health bureau regional, public health research and referral laboratory.
Dr Godana Arero (MPH, PhD in Nutritional Science) and working at Adama hospital medical college as Assi. Prof of nutrition). Mr Teklu Shiferaw (MSC, Assi Prof. working at Adama hospital medical college, Department of public health microbiology, Adama, Ethiopia.
First we thank god the Almighty for his everlasting dearest. Next, we thank Adama hospital medical college with greatly honored and privileged to acknowledge the enormous support for accomplishment of the study. We are also thankful to other line departments and individuals who played important roles in availing necessary information without which this work wouldn’t have been completed. Last, but not least, we also appreciate the contributions of all supervisors, data collectors and study participants for their respective contribution without which the work would not have been appreciate.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Arero G, Abebe A. Bacteriological quality of raw meat, antibiotics sustainability pattern of bacterial isolation and associated factors among butcher house in Adama town, Oromia Regional state, Ethiopia, 2020 AGBIR 2024;40(4):1236-1249 2020. AGBIR 2023;39(4):1-14.
Received: 27-Feb-2023, Manuscript No. AGBIR-23-90167; , Pre QC No. AGBIR-23-90167 (PQ); Editor assigned: 02-Mar-2023, Pre QC No. AGBIR-23-90167 (PQ); Reviewed: 17-Mar-2023, QC No. AGBIR-23-90167; Revised: 02-May-2023, Manuscript No. AGBIR-23-90167 (R); Published: 09-Jul-2024, DOI: 10.37532/0970-1907.24.40(4).1236-1249
