Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2024) Volume 40, Issue 5
Sweet potato is a highly valuable food crop that is widely consumed in many parts of the world. However, the skin of sweet potatoes is often discarded during consumption, even though it contains some nutrients and phytochemicals. Indeed, a study was conducted in southern Benin to evaluate the nutritional potential, antioxidant activity and phenolic compounds of the five most commonly grown sweet potato varieties. Spectrophotometric methods were used to measure the levels of Total Polyphenols (TP) and Total Flavonoids (TF). The antioxidant activity was assessed using 2,2-diphenyl-1-picryl-hydrazyl.
The results showed that TP content ranged from 2.93 ± 0.03 to 7.95 ± 0.50 mg Gallic Acid Equivalent (GAE)/g in the flesh samples and from 3.52 ± 0.01 to 7.88 ± 0.35 mg GAE/g in the whole sweet potatoes (flesh+skin). TF content ranged from 20.48 ± 0.39 to 36.64 ± 0.11 mg/g in the flesh samples and from 21.00 ± 0.71 to 40.33 ± 0.82 mg/g in the flesh+skin.
Among the different sweet potato varieties studied, 'Deux couleurs' and 'Vobodouaho' had the highest levels of phenolic compounds in their flesh. For whole samples, the 'Deux couleurs and Djowamon' varieties had the highest content of total phenolic compounds. Additionally, sweet potato samples with colored skin (red or purple) had higher TF content compared to those with white skin.
In terms of antioxidant activity, the 'Deux couleurs' variety exhibited the highest activity with an Inhibitory Concentration (IC50) of 4.76 mg/ml, while the 'Vobodouaho' variety had an IC50 of 51.4 mg/ml.
Overall, the study suggests that 'Deux couleurs' and 'Djowamon' varieties have the potential to contribute significantly to food and nutritional security, as well as the prevention of chronic diseases.
Antioxidants; Nutritional composition; Phenolic compounds; Sweet potatoes
Sweet potato (Ipomoea batatas Lam) is a widely consumed root and tuber plant, with over two billion people worldwide including it in their diets [1]. It is ranked fifth among food crops in developing countries, following rice, wheat, maize and cassava [2]. In 2021, global sweet potato production reached an estimated 88.9 million tons, grown in 115 countries. Africa alone produced around 29 million tons, with Benin contributing 53,894 tons. Sweet potato is not only important socio-economically but also culturally [2,3]. There are various sweet potato varieties worldwide, differing in flesh color, ranging from white to purple, orange and yellow [4]. Phytochemical studies have revealed that sweet potatoes contain compounds such as polyphenols, vitamins, β-carotene, amino acids, dietary fiber, potassium, copper, manganese and iron. These nutritional components, along with low lipid and cholesterol content, contribute to the potential health benefits of sweet potatoes, including anti-cancer, anti-inflammatory, anti-tumor, anti-diabetic, anti-hemorrhoidal and cardiovascular disease prevention properties [5-9].
Sweet potatoes are particularly valuable in combating malnutrition and improving food security in developing countries due to their adaptability to marginal lands and rich nutritional content [10]. Despite their relatively high carbohydrate content, sweet potatoes have been recognized by the World Health Organization (WHO) for their anti-diabetic activity [11,12]. Previous studies have shown that sweet potatoes can help stabilize blood sugar levels and reduce insulin resistance [6].
It is worth noting that the skin of sweet potatoes contains important nutrients and phytochemicals, although it is often discarded before consumption in many regions [13]. In addition to their nutritional significance, sweet potatoes play a significant role in the economic well-being of farmers and the entire value chain. In Benin, sweet potatoes are consumed in cooked or fried form at the household level, especially during lean periods. They also contribute to combating malnutrition in children, particularly varieties with colored flesh rich in β-carotene, which are used in infant flour [7,12].
Despite its numerous benefits, the industrial transformation of sweet potatoes is limited in Benin, despite its potential as a source of starch and food color. Previous studies have identified 87 local varieties in South Benin, but the sweet potato remains underutilized, with limited scientific research conducted on it [14]. Therefore, it is important to study the nutritional and physico-chemical composition of sweet potato varieties in Benin to unlock its full potential for nutrition, therapy and potential industrial applications [15]. The present work aims to assess the physico-chemical and nutritional potential of the five most cultivated sweet potato varieties in South Benin.
Reagents
Following chemicals were purchased from Sigma chemical (Darmstadt, Germany): 2,2-Diphenyl-1-Picrylhydrazyl (DPPH), Potassium Hexacyanoferrate (K3[Fe(CN)6]), trichloroacetic acid, gallic acid, ascorbic acid, quercetin and Hexachloroferrate (FeCl6). Additionally, Folin-Ciocalteu phenol reagent, anhydrous Sodium Carbonate (Na2CO3), aluminum chloride, potassium acetate and solvent methanol were obtained from Merck chemical supplies (Darmstadt, Germany).
Sample collection and preparation
The present study was carried out on the flesh and flesh+skin of five varieties (the most cultivated varieties in South Benin), giving ten samples of sweet potatoes (Ipomoea batatas Lam). The sweet potatoes were collected from four different departments (Oueme, Plateau, Atlantique and Littoral) known for their high production in South Benin [15]. The specific characteristics and areas of harvest are provided in Table 1.
| Abbreviation | Skin color | Pulp color | Harvest location |
|---|---|---|---|
| DC | Red (dark purple) | Yellow | Pahou, Dantopka market |
| DJ | Red | White | So-ava, Sissepka market, Akassato market, Sakete |
| MC | White | Yellow-whitish | Sokan, Soava market, Sissèpka, Azowlisse |
| BW | White | Yellow | Dantokpa market, Azowlissé market |
| VO | White | White | Dantokpa market, Azowlissé Market |
Note: DC: Deux Couleurs; DJ: Djowamon; MC: Meché; BW: Bombo WéWé; VO: Vobodouaho.
Table 1: Characteristics and places of harvest of sweet potato samples.
Upon arrival at the laboratory, the harvested sweet potato tubers were washed with tap water and divided into two batches. The first batch was peeled using a manual peeler and cut into slices with a thickness of 4 mm. The second batch, which included both the skin and flesh, was also cut into 4 mm thick slices. Both batches were then dried using a ventilated dryer at a temperature of 50°C for a duration of 48 h. After drying, the sweet potato slices were milled until a flour with a granulometry of approximately 500 μm was obtained. The resulting flours from the different samples were then assigned unique codes and packaged in glass bottles for storage and future use.
Chemical and nutritional composition
Chemical and nutritional composition of the samples was determined using the Association of Official Analytical Chemists (AOAC) official method for nutrient analysis. The moisture content, crude ash content, protein, fat and fiber were analyzed following the AOAC method. The carbohydrate content was calculated using the subtraction method:
%carbohydrates = 100 - (%moisture+ %fat + %protein + %ash)
Evaluation of sweet potato color
Color is a very important sensory attribute in the appreciation and choice of food products. The various color measurements were performed by Chroma meter, based on the color parameters of the International Commission on Illumination (CIE). CR-410 chroma meter was used to determine the color of the skin and flesh of fresh and cooked sweet potatoes. The results represent the average of 10 measurements from each sample. The color variation ΔE of the skin and flesh of the different varieties of fresh and cooked sweet potato was calculated from the white porcelain reference plate (L*=94.7, a*=-0.26, b*=3.66) according to the formula:

ΔE indicates the color variation between sweet potato sample and white plate. Lt*, at* and bt* refer to the parameters of the white plate and Li*, ai* and bi* refer to the parameters of the sweet potato samples.
Phytochemical screening
The aim of phytochemical screening is to detect the presence of various phytochemicals in sweet potato samples. The main groups of interest include alkaloids, free anthracenes, anthocyanins, leucoanthocyanins, flavonoids, coumarins, saponins, tannins, triterpenes and reducing compounds. Two complementary methods, namely the tube test method and the Column Chromatography Method (CCM) were employed for this purpose.
Total polyphenol content
The total polyphenol content of sweet potato extracts was determined using a spectrophotometric method [16]. To perform the analysis 0.2 ml of each sweet potato extract (at a concentration of 1 mg/ml) was mixed with 0.8 ml of Folin-Ciocalteu (10%) and incubated for 4 min. Then, 1 ml of sodium carbonate solution (75 g/l) was added to the mixture. The resulting mixture was incubated at room temperature in the dark for 2 h. The absorbance of each reaction mixture was measured at 765 nm using a spectrophotometer in triplicate. A blank consisting of a mixture of 1 ml Folin and 1 ml sodium carbonate was used for comparison. The results were expressed as milligram (mg) gallic acid equivalent per gram (g) of extract (mg GAE/g extract).
Total flavonoid content
The total flavonoid content of sweet potato samples was determined using the aluminum chloride colorimetric procedure [16]. In this method 0.5 ml of each sweet potato extract (at a concentration of 1 mg/ml) was mixed with 1.5 ml of methanol, 0.1 ml of potassium acetate (1 M) and 0.1 ml of Aluminum Trichloride (AlCl3) solution (10%). Then, 2.8 ml of distilled water was added to the mixture. After a 30-min incubation period, the absorbance of the reaction was measured at 415 nm using a spectrophotometer. A blank consisting of 1 ml of methanol and 1 ml of distilled water was used for comparison. The results were expressed as milligram Quercetin Equivalents (mgQE) per gram (g) of extract. The measurement was performed in triplicate and the reported values represent the average of three replicates.
Evaluation of antioxidant activity by inhibition of 2,2-diphenyl-1-picrylhydrazyl
To evaluate the antioxidant activity of sweet potato extracts, the DPPH assay was employed. The tube method was used to determine the 0.002% scavenging activity of 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) radical in the sweet potato samples [17]. Various concentrations of plant extracts ranging from 1 mg/ml to 0.78 mg/ml were tested. The tubes containing the samples were incubated in a dark room at 25°C for 20 min. After incubation, the Optical Density (OD) of each solution was measured at 517 nm using a spectrophotometer. The tests were conducted in triplicate. As a positive control, ascorbic acid was used.
Statistical analysis of results
The results obtained from triplicate experiments during our study were collected and analyzed by Analysis of Variance (ANOVA); Statistical Package for the Social Sciences (SPSS) 16.0. (SPSS Inc, USA). The statistical analysis of the results obtained during the determination of the phenolic compounds and the nutritional composition was carried out using the student’s test which allowed to calculate the Probability (P) value for which a result presents a significant difference or not with a threshold of 5% (p<0.05).
Physicochemical and nutritional analysis of sweet potato varieties
Chemical composition and nutritional value of sweet potato: Table 2, presents the chemical and nutritional composition, including moisture, ash, carbohydrate, fat, protein, fiber and energy value of different sweet potato varieties with and without skin. The moisture content of the sweet potato varieties ranged from 63.0 ± 0.22% to 70.00 ± 0.21%. Some varieties without skin had higher moisture content compared to those with skin, except for Djowamon Without Skin (DJOS) and Meché Without Skin (MCOS). Previous studies have reported higher moisture content than our results, which could be due to factors such as variety, geographical area, climate, light exposure, soil and genetic composition [18,19]. Sweet potatoes have a high moisture content compared to other roots and tubers, making them perishable and challenging to preserve. However, our samples had lower moisture content compared to other research findings, indicating a higher content of dry matter and condensed nutrients.
| Samples | Moisture content | Ash (%) | Carbohydrate (%) | Fat (%) | Protein (%) | Fiber (%) | Flour yield | Energy value (Kcal/100 g) |
|---|---|---|---|---|---|---|---|---|
| Without skin | ||||||||
| DCOS | 70.00 ± 0.21a | 2.88 ± 0.05a | 72.30 ± 0.12b | 0.60 ± 0.23a | 4.39 ± 0.44b | 2.61 ± 0.23b | 32.07 | 312.16 |
| DJOS | 63.27 ± 0.99c | 2.02 ± 0.19a | 72.28 ± 0.34c | 0.32 ± 0.21b | 4.37 ± 0.27b | 3.03 ± 0,08b | 35.5 | 309.48 |
| MCOS | 63.53 ± 0.10c | 2.14 ± 0.51a | 74.19 ± 0.03b | 0.41 ± 0.67b | 3.73 ± 0.27c | 2.18 ± 0.06c | 36.06 | 315.37 |
| BWOS | 67.33 ± 0.15b | 1.77 ± 0.02c | 71.64 ± 0.14c | 0.38 ± 0.23b | 3.94 ± 0.33c | 2.80 ± 0.38b | 37.88 | 305.74 |
| VOOS | 65.47 ± 0.29ab | 1.41 ± 0.06c | 71.81 ± 0.67c | 0.2 ± 0.89b | 6.74 ± 0.56a | 1.89 ± 0.22c | 34.32 | 316.00 |
| With skin | ||||||||
| DCWS | 67.47 ± 0.29b | 2.27 ± 0.07a | 75.75 ± 0.32a | 0.40 ± 0.34b | 7.88 ± 0.97a | 3.18 ± 0.52b | 46.05 | 338.12 |
| DJWS | 67.33 ± 0.15b | 1.65 ± 0.05b | 77.09 ± 0.19a | 0.39 ± 0.25b | 5.18 ± 0.87b | 4.82 ± 0.24a | 37.51 | 332.59 |
| MCWS | 64.37 ± 0.20c | 2.73 ± 0.08a | 72.9 ± 0.23c | 0.29 ± 0.99b | 4.37 ± 0.55c | 3.61 ± 0.07b | 37.51 | 311.69 |
| BWWS | 64.33 ± 0.53c | 1.92 ± 0.01b | 76.61 ± 0.02a | 0.37 ± 0.32b | 4.28 ± 0.67c | 3.16 ± 0.14b | 47.22 | 326.89 |
| VOWS | 63.07 ± 0.22c | 1.83 ± 0.01b | 74.00 ± 0.43b | 0.40 ± 0.88b | 7.64 ± 0.77a | 2.59 ± 0.43b | 43.01 | 330.16 |
Note: Values in the same column are mean ± standard deviation (n=3); data in same column with different letters are significantly different (p<0.05), DCOS: Deux Couleurs Without Skin; DJOS: Djowamon Without Skin; MCOS: Méché Without Skin; BWOS: Bombo WéWé Without Skin; VOOS: Vobodouaho Without Skin; DCWS: Deux Couleurs With Skin; DJWS: Djowamon With Skin; MCWS: Méché With Skin; BWWS: Bombo WéWé With Skin; VOWS: Vobodouaho With Skin.
Table 2: Chemical and nutritional composition of sweet potato varieties studied.
Carbohydrates were the predominant component in the sweet potato varieties, ranging from 71.64 ± 0.14% to 77.09 ± 0.19%. Flesh+skin samples generally had higher carbohydrate content compared to flesh samples, except for MCOS. Previous studies have reported higher carbohydrate content in sweet potato flour than our findings variation of 84.16% to 94.8% (dry weight), while others have reported lower values 65.59% to 48.35% [20,21]. These variations could be attributed to factors such as variety harvest season and tuber maturity stages.
Protein content ranged from 3.73 ± 0.27% to 7.88 ± 0.97%. Flesh+skin samples generally had significantly (p<0.05) higher protein content compared to flesh samples. Protein content in sweet potato varieties from Sri Lanka was lower than our findings [22]. Compared to other roots and tubers like cassava (0.87%), sweet potatoes have a higher protein content [6].
The fat content of sweet potato varieties ranged from 0.2 ± 0.89% to 0.60 ± 0.23%. The Deux Couleurs With Skin (DCWS) sample had the highest lipid content. Sweet potatoes are known for their low-fat content and our results were consistent with previous studies [23]. However, Tumuhimbise et al., found a fat content of 0.17%, which was lower than our results [24].
Ash content ranged from 1.41 ± 0.06% to 2.88 ± 0.05%. The Deux Couleurs Without Skin (DCOS) sample had the highest ash content. Our results were similar to those obtained by Ladoh et al., for nine varieties in Bangladesh [17]. Goodbody also reported the same total ash content in sweet potato at 1.7% [25]. In contrast, the results of Rose et al., were between 0.40% and 0.44%, which is lower than our results [26]. This observed difference in ash content could be due to soil types or climatic conditions.
Crude fiber content in the sweet potato varieties ranged from 1.89 ± 0.22% to 4.82 ± 0.24%. The Djowamon with Skin (DJWS) sample had the highest fiber content. Flesh+skin samples generally had higher fiber content than flesh samples, indicating that the skin of sweet potatoes is rich in fibers. Fiber content varied in different studies, with some reporting higher levels. Studies by Oomen et al., showed a fiber content of 3.9%, which is similar to the results of our study [27]. On the other hand, Ishida et al., and Ahmed et al., have found high levels ranging from 5.26% to 7.14% and from 2.28% to 11.7% respectively [28,29]. However, Rose et al., found 0.11% to 0.14% in Rwandan varieties [26]. Dietary fiber is important for preventing and treating chronic diseases such as colon cancer, diabetes, heart disease and certain diseases of the digestive tract [30].
The energy values of the sweet potato samples ranged from 309.37 to 337.12 Kcal/100 g. Flesh+skin samples had higher energy values and flour yields compared to flesh samples, primarily due to their carbohydrate, protein and fiber content. These results highlight the nutritional composition and energy intake benefits of sweet potato peel, which could also increase the yield of commercially available flour.
Color of fresh and cooked sweet potato varieties:Color is a significant sensory attribute for characterizing, appreciating and selecting foods, as well as judging their qualities. Tables 3 and 4, present the skin and flesh color parameters of the various varieties of fresh and cooked sweet potatoes that were studied. The skin color of sweet potatoes (both fresh and cooked) ranges from white and creamy white to red and purple. The flesh color of sweet potatoes (both fresh and cooked) varies depending on the variety, ranging from white and creamy white to pale yellow and dark yellow. Among the varieties, Meché (MC), Vobodouaho (VO) and Bombo WéWé (BW) had the highest L* values for both fresh and cooked sweet potato skins. This was evident in Figure 1, where these three varieties appeared white compared to Deux Couleurs (DC) and Djowamon (DJ), which had red to purple skin. The ΔE values also support these observations, with DC and DJ having significantly higher values of 54.06 and 47.09, respectively, compared to BW (33.51), VO (34.30) and MC (28.91). The high ΔE value indicates a significant difference (p<0.05) in skin color. Additionally, a significant increase (p<0.05) in the color parameter (L*) was observed for the flesh of all sweet potato varieties, suggesting that the flesh is lighter in color compared to the skin across all varieties. Therefore, these findings indicate that the skin color of sweet potato varieties is not correlated with the flesh color. When sweet potatoes are cooked, there is a decrease in the color parameters (L*) of both the skin and flesh, resulting in a loss of whiteness. These results align with the observations made by Kusuma et al., [31]. However, the changes in the values of a* and b* do not follow the same pattern as the L* value. Cooking leads to a decrease in the a* value of the flesh for all varieties, while the b* value increases, except for the DJ and MC varieties. This increase in the b* value is also evident in Figure 1, where the flesh of the cooked DC, BW and VO varieties appears more yellow. The color of fresh and cooked sweet potato varieties is likely influenced by natural pigments, which can impact the red-yellow index of the sweet potato. These natural pigments may also have nutritional significance for sweet potatoes [32].
| Flesh | Skin | |||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | L* | a* | b* | ΔE | |
| DC | 82.9 ± 0.33b | -0.03 ± 0.21a | 33.1 ± 0.74a | 31.67 | 43.6 ± 0.10d | 17.5 ± 019a | 3.64 ± 1.44d | 54.06 |
| DJ | 80.7 ± 0.61c | 0.50 ± 0.41a | 15.2 ± 0.38d | 18.12 | 50.3. ± 1.54c | 13.2 ± 0,57b | 11.9 ± 0.05c | 47.09 |
| MC | 85.7 ± 0.20a | -3.57 ± 0.18c | 28.9 ± 0.10b | 27.03 | 73.4 ± 0.90a | -3.88 ± 0.26d | 22.85 ± 0,10a | 28.91 |
| BW | 82.9 ± 0.01b | -1.43 ± 0.23b | 30.6 ± 0.21b | 33.12 | 66.3 ± 0.16b | 5.13 ± 0.08c | 20.58 ± 0.08b | 33.51 |
| VO | 86.4 ± 0.12a | -1.64 ± 0.40b | 22.1 ± 0.53c | 20.25 | 68.6 ± 1.42b | 3.81 ± 0.47c | 25.58 ± 1.55a | 34.3 |
| White plate | 94.7 | -0.26 | 3.66 | - | 94.7 | -0,26 | 3,66 | - |
Note: Values in the same column are mean ± standard deviation (n=3); data in same column with different letters shows significant differences (p<0.05); L*: Lightness value; a*: red-green chroma; b*: yellow-blue chroma; ΔE: colour variation between sweet potato sample and white plate; DC: Deux Couleurs; DJ: Djowamon; MC: Meché; BW: Bombo WéWé; VO: Vobodouaho.
Table 3: Skin and flesh colour parameters of fresh sweet potato varieties.
| Flesh | Skin | |||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | L* | a* | b* | ΔE | |
| DC | 68.20 ± 0.68c | -3.49 ± 0.13b | 35.69 ± 1.41a | 40.93 | 40.36 ± 0.67d | 6.76 ± 0.21a | 3.89 ± 0.31d | 54.8 |
| DJ | 75.14 ± 0.18a | -1.99 ± 0.03a | 11.60 ± 0.05c | 21.18 | 43.46 ± 2.78d | 7.18 ± 0.68a | 6.75 ± 0.50c | 51.87 |
| MC | 75.77 ± 0.30a | -4.34 ± 0.04c | 22.88 ± 0.37b | 27.29 | 61.83 ± 1.17a | 1.71 ± 0.58c | 17.6 ± 0.31a | 35.77 |
| BW | 71.54 ± 0.74b | -3.49 ± 0.13b | 34.28 ± 1.34a | 38.53 | 48.37 ± 0.33c | 1.37 ± 0,58c | 12.2 ± 0.30b | 47.15 |
| VO | 71.47 ± 0.33b | -3.93 ± 0.21b | 25.42 ± 0.38b | 32.04 | 54.50 ± 1.43b | 2.85 ± 0.68b | 16.3 ± 1.19a | 42.25 |
| White plate | 94.7 | -0.26 | 3.66 | - | 94.7 | -0.26 | 3.66 | - |
Note: Values in the same column are means ± standard deviation (n = 3); data in same column with different letters are significantly different (p<0.05); L*: Lightness value; a*: red-green chroma; b*: yellow-blue chroma; ΔE: colour variation between sweet potato sample and white plate; DC: Deux Couleurs; DJ: Djowamon; MC: Meché; BW: Bombo WéWé; VO: Vobodouaho.
Table 4: Skin and flesh colour parameter of cooked sweet potato varieties.
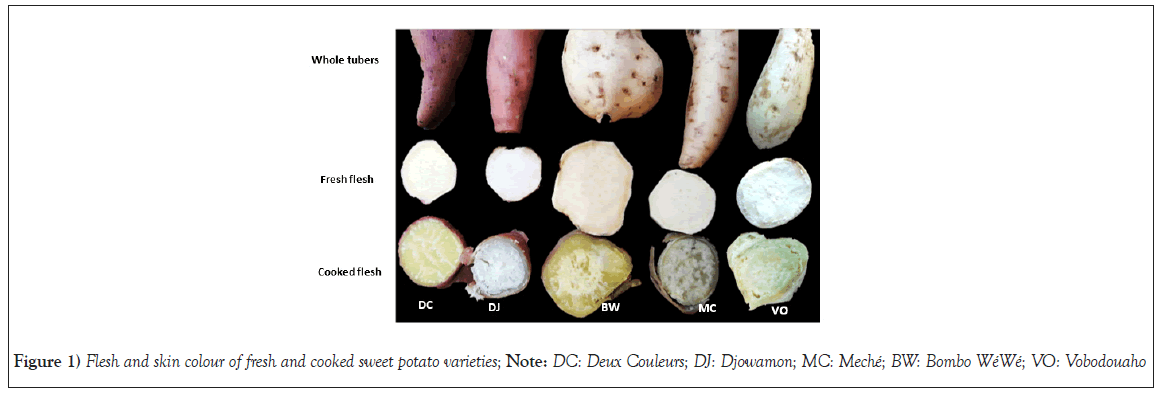
Figure 1: Flesh and skin colour of fresh and cooked sweet potato varieties; Note: DC: Deux Couleurs; DJ: Djowamon; MC: Meché; BW: Bombo WéWé; VO: Vobodouaho
Phytochemical screening
Phytochemical study conducted on different varieties of sweet potatoes (flesh and skin+flesh) involved the use of Thin Layer Chromatographic (TLC) analysis. The TLC analysis showed the presence of alkaloids and flavonoids, which were indicated by blue spots and green or blue fluorescence under Ultraviolet (UV) light, respectively. The results of the phytochemical screening revealed the presence of various secondary metabolites in the aqueous extracts of both flesh and flesh+skin samples, including saponosides, free anthracenes, flavonoids, leucoanthocyanins, mucilages, coumarins, reducing compounds, alkaloids, steroids and triterpenes (Table 5). It is worth noting that tannins, anthocyanins and quinonic derivatives were absent in all samples. These findings align with previous reports [33]. Another study on sweet potato leaves also found similar secondary metabolites, except for tannins [34]. Additionally, Panda et al., identified the same phytochemicals in leaf extracts, but with the presence of tannins and the absence of alkaloids and triterpenes [35]. These variations could be attributed to the sweet potato variety or organ, as well as the solvents used in the extractions.
Determination of phenolic compounds
| Phytochemical components | Without skin | With skin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DCOS | DJOS | MCOS | BWOS | VOOS | DCWS | DJWS | MCWS | BWWS | VOWS | |
| Saponin | + | + | + | + | + | + | + | + | + | + |
| Free anthracene | + | + | + | + | + | + | + | + | + | + |
| Tannin | - | - | - | - | - | - | - | - | - | - |
| Flavonoid | + | + | + | + | + | + | + | + | + | + |
| Anthocyanin | - | - | - | - | - | - | - | - | - | - |
| Leuco anthocyanin | + | + | + | + | + | + | + | + | + | + |
| Quinonics | - | - | - | - | - | - | - | - | - | - |
| Mucilages | + | + | + | + | + | + | + | + | + | + |
| Coumarin | + | + | + | + | + | + | + | + | + | + |
| Reducing compound | + | + | + | + | + | + | + | + | + | + |
| Triterpene | + | + | + | + | + | + | + | + | + | + |
| Alkaloids | + | + | + | + | + | + | + | + | + | + |
Note: (-): Absence; (+): Presence; DCOS: Deux Couleurs Without Skin; DJOS: Djowamon Without Skin; MCOS: Méché Without Skin; BWOS: Bombo WéWé Without Skin; VOOS: Vobodouaho Without Skin; DCWS: Deux Couleurs With Skin; DJWS: Djowamon With Skin; MCWS: Méché With Skin; BWWS: Bombo WéWé With Skin; VOWS: Vobodouaho With Skin.
Table 5: Qualitative phytochemical constituents of different sweet potato samples.
Total polyphenol content: Total Polyphenol (TP) content was determined in samples of sweet potato varieties (flesh and flesh+skin) using a standard curve (y=0.043 × -0.0513, R2=0.994), created with gallic acid. The total polyphenol levels were depicted in Figure 2A. In the flesh samples of different sweet potato varieties, the TP content ranged from 2.93 ± 0.03 to 7.95 ± 0.5 mg (GAE)/g, while in the flesh+skin samples, it ranged from 3.52 ± 0.01 to 7.88 ± 0.35 mg (GAE)/g. These levels were lower than those found in the flesh of 40 Chinese sweet potato varieties, which ranged from 2.73 ± 0.02 to 12.46 ± 0.62 g/100 g [36]. Similarly, in 05 varieties of sweet potatoes grown in the Philippines, the TP content ranged from 192.7 to 1159.0 mg GAE/100 g of dry sample [37]. However, our results were higher than those obtained in 14 sweet potato genotypes, which ranged from 1.4 mg/g to 4.7 mg/g [9]. These variations in TP content could be attributed to the different sweet potato varieties or the solvents used for extraction. Among the flesh samples, the Vobodouaho Without Skin (VOOS), DCOS and DJOS varieties exhibited the highest levels of total polyphenols, with values of 7.95 ± 0.5, 7.53 ± 0.36 and 6.72 ± 0.06 mg GAE/g, respectively. For the flesh+skin samples, the highest polyphenol contents were observed in the Vobodouaho With Skin (VOWS), Bombo WéWé With Skin (BWWS) and Méché With Skin (MCWS) varieties, with values of 7.88 ± 0.35, 7.64 ± 0.76 and 5.93 ± 0.22 mg GAE/g, respectively. A comparative analysis of the flesh and flesh+skin samples revealed that the BWWS and MCWS samples, which had low levels of polyphenols in their flesh, exhibited higher levels in the flesh+skin. These findings suggest that polyphenols are concentrated in the flesh of certain sweet potato varieties (VOOS, DCOS and DJOS), while in others (BWWS and MCWS), they are more abundant in the skin. The variation in total polyphenols may depend on the sweet potato variety, color and different parts of the tuber. Musilová et al., demonstrated significant differences in total polyphenols between the O'Henry (white), Beauregard (orange) and 414 (purple) varieties, as well as variations between the flesh and skin within the same variety (polyphenol skin/flesh polyphenol=4.27 for Beauregard) [38]. These results suggest that consuming sweet potatoes with the skin on may be beneficial.

Figure 2: A): Total polyphenol Content; B): Total flavonoid contents; Note: DCOS: Deux Couleurs Without Skin; DCWS: Deux Couleurs With Skin; DJOS: Djowamon Without Skin; DJWS: Djowamon With Skin; MCOS: Méché Without Skin; MCWS: Méché With Skin; BWOS: Bombo WéWé Without Skin; BWWS: Bombo WéWé With Skin; VOOS: Vobodouaho Without Skin; VOWS: Vobodouaho With Skin
Total flavonoid contents: Total flavonoid contents were determined in samples of sweet potato varieties (flesh and flesh+skin) using a standard curve generated with quercetin (y=0.325 × -0.363, R2=0.995). Figure 2B, illustrates the flavonoid content in the flesh and flesh+skin samples of different sweet potato varieties. The total flavonoid content ranged from 20.48 ± 0.39 to 36.64 ± 0.11 mg/g for the flesh samples and from 21.00 ± 0.71 to 40.33 ± 0.82 mg/g for the flesh+skin samples. DCOS and Bombo WéWé Without Skin (BWOS) flesh samples exhibited the highest flavonoid content, measuring 36.64 ± 0.11 and 35.30 ± 0.78 mg/g, respectively, with a significant difference (p<0.05). VOOS, MCOS and DJOS samples showed flavonoid contents of 31.49 ± 0.94, 30.67 ± 0.64 and 20.48 ± 0.39 mg/g, respectively. In contrast, for the flesh+skin samples, DCWS and DJOS displayed high levels of flavonoid content, measuring 40.33 ± 0.82 and 33.34 ± 0.28 mg/g, respectively, followed by BWWS (25.42 ± b0.36), MCWS (23.36 ± 0.16) and VOWS (21.00 ± 0.71). The high flavonoid content observed in DCWS and DJWS samples may be attributed to the red and purple color of their skin, while the content in DCOS and BWOS samples could be linked to the yellowish color of their flesh. Previous studies have highlighted the importance of flavonoids in sweet potatoes, as they contribute to pigmentation and overall well-being [39]. Comparing the flesh and flesh+skin samples, a significant difference (p<0.05) in total flavonoid content was observed. Another study reported total flavonoid levels ranging from 0.60 to 17.83 g EQ/100 g in four varieties of sweet potato tubers, which were lower than the results obtained in this study [40]. However, the authors found higher total flavonoid levels (ranging from 15.43 to 59.79 g EQ/100 g) in sweet potato leaves compared to our findings. Additionally, research has shown that the ethanolic extract from yellow-fleshed sweet potato leaves has a higher total flavonoid content compared to extracts from other varieties. The variations in flavonoid content among different samples could be attributed to the sweet potato variety, skin color, flesh color or the solvent used for extraction.
Antioxidant activity by 2,2-diphenyl-1-picrylhydrazyl inhibition
The antioxidant activity of the different sweet potato extracts was evaluated using IC50, which represents the concentration of extract needed to neutralize or reduce 50% of the free radical. A lower IC50 value indicates a higher antioxidant potential. The results of the antioxidant activity for the flesh and flesh+skin of various sweet potato varieties, expressed in IC50 are presented in Table 6. For the flesh samples, the IC50 values ranged from 6.97 to 51.41 mg/ml, with the DCOS and DJOS varieties showing the highest inhibition percentages at IC50 values of 6.97 and 11.57 mg/ml, respectively. The MCOS, BWOS and VOOS varieties followed with IC50 values of 14.07, 17.57 and 51.41 mg/ml, respectively. In contrast, the IC50 values for the flesh+skin samples were 4.76 mg/ml (DCWS), 9.64 mg/ml (DJWS), 10.38 mg/ml (MCWS), 13.99 mg/ml (BWWS) and 25.23 mg/ml (VOWS). It is worth noting that all flesh+skin samples had lower IC50 values compared to the flesh samples, with DCWS (4.76 mg/ml) and DJWS (9.64 mg/ml) exhibiting the lowest IC50 values (highest inhibitory percentage). These results indicate that the skins of sweet potato varieties contain antioxidant compounds. The purple to red skin color of the DCWS and DJWS varieties is believed to contribute to their high antioxidant activity. These findings are consistent with previous research that demonstrated a high antioxidant potential in sweet potato skins, particularly those with purple and white skin, with higher activity observed in purple skins [12]. The study also found that purple peels had higher antioxidant potential compared to white flesh. Another study on different sweet potato cultivars revealed that varieties with colored skin exhibited high antioxidant activity [41]. In the Philippines, the light purple flesh variety 'Dakol' displayed the highest antioxidant activity, while the white flesh variety 'Emelda' had the lowest [37]. Additionally, some authors reported that among orange, yellow, white and purple-fleshed varieties, the purple-fleshed ones exhibited the highest antioxidant activity [38,41]. These observations suggest that color plays a role in the antioxidant activity of sweet potato varieties. The high antioxidant activity observed in the DCOS, DJOS, DCWS and DJWS samples could be attributed to their high levels of total phenolic compounds in both the flesh and skin. This study establishes a correlation between total phenolic content and antioxidant activity in different sweet potato varieties. Several studies have also highlighted the relationship between high levels of phenolic compounds and antioxidant activity in sweet potato tubers and leaves [41,42]. However, despite the relatively high phenolic content in VOOS and VOWS, these compounds exhibited low antioxidant activity. This discrepancy could be attributed to the nature and structure of the polyphenols present in sweet potato. Studies conducted in Indonesia on the antioxidant activity of different sweet potato varieties demonstrated that varieties with high polyphenol content had low antioxidant activity [39]. The authors also found that glycosylated flavonoids had lower antioxidant activity compared to non-glycosylated flavonoids. Furthermore, only flavonoids with specific Hydroxyl (OH) positions exhibited high antioxidant activity compared to other flavonoids with different positions [43].
| Samples | PI (%) | IC50 (mg/ml) |
|---|---|---|
| Without skin | ||
| DCOS | 72.36 | 6.97 |
| DJOS | 41.78 | 11.57 |
| MCOS | 29.71 | 14.07 |
| BWOS | 19.49 | 17.57 |
| VOOS | 8.14 | 51.41 |
| With skin | ||
| DCWS | 78.5 | 4.76 |
| DJWS | 60.9 | 9.64 |
| MCWS | 50.91 | 10.38 |
| BWWS | 38.00 | 13.99 |
| VOWS | 14.10 | 25.23 |
Note: PI: Percentage Inhibition; IC: Inhibitory Concentration; DCOS: Deux Couleurs Without Skin; DJOS: Djowamon Without Skin; MCOS: Méché Without Skin; BWOS: Bombo WéWé Without Skin; VOOS: Vobodouaho Without Skin. DCWS: Deux Couleurs With Skin; DJWS: Djowamon With Skin; MCWS: Méché With Skin; BWWS: Bombo WéWé With Skin; VOWS: Vobodouaho With Skin.
Table 6: Percentage Inhibition (PI) and Inhibitory Concentration (IC50) of different sweet potato extracts.
The nutritional compositions of the sweet potato varieties studied were generally similar, except for 'Deux Couleurs' (DC) and 'Djowamon' (DJ), which had higher levels of carbohydrates, ash contents, protein and fiber. These two varieties, with purple and red skin respectively, also exhibited higher levels of total polyphenols and flavonoids compared to the other varieties. As a result, they showed significantly higher antioxidant activities. Furthermore, the phenolic and nutrient compounds were found to be more concentrated in the skin of certain varieties, particularly those with colored flesh. Therefore, it is recommended to consume sweet potatoes with the skin intact. The elevated antioxidant activity observed in the study is likely attributed to the high content of total phenolic compounds. These findings highlight the potential and characteristics of sweet potato varieties, especially those with purple and red skin, for their nutritional and therapeutic functions. It is suggested that these nutrient-rich varieties, with their abundance of phenolic compounds, be further developed for improved nutritional and therapeutic management. Additionally, it would be beneficial to continue researching the therapeutic effects of the three selected varieties (DC, DJ and VO) in relation to the prevention and management of chronic diseases such as hypertension, hemorrhoids and diabetes. Further investigation is also recommended to identify the bioactive components responsible for these curative or preventive effects. The results obtained from this study can be utilized in the development of infant flours derived from sweet potato tubers with colored skin, thereby adding value to the findings.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Lagnika C. Antioxidant activity and nutritional potential of the most cultivated sweet potato varieties in Benin. AGBIR.2024;40(5):1311-1317.
Received: 07-Aug-2024, Manuscript No. AGBIR-24-144831; , Pre QC No. AGBIR-24-144831 (PQ); Editor assigned: 09-Aug-2024, Pre QC No. AGBIR-24-144831 (PQ); Reviewed: 23-Aug-2024, QC No. AGBIR-24-144831; Revised: 30-Aug-2024, Manuscript No. AGBIR-24-144831 (R); Published: 06-Sep-2024, DOI: 10.35248/0970-1907.24.40.1311-1317
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
