Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2024) Volume 40, Issue 4
A lot of bacterial resistance cases towards antibiotics, hearten numerous efforts to obtain new origin of antibiotics. Endophytic bacteria represent potential microbes capable of producing significant bioactive compounds. Psidiium guajava, Mangifera indica, Cassia occidentalis, Calotropis procera and Hibiscus rosa-sinensa have been employed as ingredients of many traditional herbal medicines. The work is aimed to scrutinize the antimicrobial and antioxidant activity of the bacterial endophytes linked with the mentioned medicinal plants. Thirty bacterial colonies were isolated and identified based on physiological and biochemical characteristics shown by the isolates, subsequently, only eight isolates were chosen for this investigation. Antimicrobial activity of the isolates was known via agar well diffusion method. The supernatant of each endophytic culture isolate was plunged on agar well against pathogenic bacteria Escherichia coli and Staphylococcus aureus. Achromobacter xylosoxidans (GL-1), Bacillus megaterium (GB-1), Pseudomonas chlororaphis (CPS-2), Citrobacter koseri (GST-1), Siccibacter colletis (COB-1), Eaerogenes and Coccobacilli (HBL-3) showed antimicrobial activity against Staphylococcus aureus, only Bacillus amyloliquefaciens (GB-3) showed resistance. While isolate Bacillus amyloliquefaciens (GB-3) and E. aerogenes CPF-1 showed antimicrobial activity against Escherichia coli, whereas Achromobacter xylosoxidans (GL-1), Bacillus megaterium (GB-1), Pseudomonas chlororaphis (CPS-2), Siccibacter colletis (COB-1) and Coccobacilli (HBL-3) showed resistance against Escherichia coli respectively. All selected endophytic isolates showed antioxidant activity as observed via scavenging capability toward 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) radical with successive percentage Achromobacter xylosoxidans GL-1 (92.55%), Bacillus megaterium (GB-1) 93.72, Pseudomona schlororaphis (CPS-2) 90.84%, Bacillus amyloliquefaciens (GB-3) 93.50%, Citrobacter koseri (GST-1) 92.76%, Siccibacter colletis (COB-1) 93.18%, E. aerogenes (CPF-1) 90.41% and Coccobacilli (HBL-3) 92.01%.
Endophytes; Antimicrobial; Antioxidant; Plants; Secondary metabolites
Traditionally plants are regarded as medicinal source used by ancient man millennia ago. It is known to date that extracts from plants in crude form are functioned in curing human diseases. They are considerably important due to the fact that various bioactive compounds are sourced from them. In addition, the medicinal features of the plants are linked to the biologically-active molecules within the plant, such as Anthraquinones (chrysophanol, emodin and physcion), phenolics, terpenes, rotenoids, steroids (carpesterol, ecdysterone and β-sitosterol) and saponins [1]. And they are said to be produced either by the plants or by the microbial community residing asymptomatically in any part of the plant region. Yadav et al., [2] concluded that the important molecules responsible for curing diseases in the plants are synthesis of endophytes. Independently endomicrobiome are capable of producing array of bioactive compounds [3]. Generally, this process of unobservable and harmless microbial dwelling within plant tissues is what is said to be endophytism and the dwellers as endophytes. Certainly, endophytic microbes are harmless in relationship with their host plant [4]. The dwelling relationship of the microbes is non-specific and non-selective, meaning all part of the plant could be colonized being it above ground or below ground region. So, its ubiquitous in nature. Most of the tissues of the plants are colonized by endophytes that helps in secreting secondary metabolites aim to control the metabolism of the plant, even at very stressful situation [5].
Primarily, considering the pharmacological perspective, endophytes are highly regarded as an essential source of newly potential compounds based on the chemical diversity seen in them, as well as production of numerous crucial metabolites that could serve as medicine [6]. Endophytes produce divergent secondary metabolites with reliable compounds leading to discovery of new drugs [7]. Knowing and uncovering of natural products by endophytes has been marked to be progressively beneficial, thereby new chiefly alternative which is less harming for drug formulation could attain. Among the diverse efficacy demonstrated by endophytes due to their ability of producing many potent metabolites are being antiparasitic, antimicrobial, antioxidant, immunosuppressant, insulin mimetic and neuroprotective [8]. Investigation for potent-producing endophytes has been profitable, as several reports confirm the production of ease and lifesaving molecules by endophytes. The biosynthesis of Paclitaxel (TaxolO) by endophytes which is known to be plant metabolite as an anticancer agent made them highly recognized and economically viable. Reports by Gill et al., [9] also affirm the production of paclitaxel by endophytes.
Antimicrobial activity is among the most important beneficial bioactivities possess by endophytes. And it’s said to be the most predominant vital compounds biosynthesized [10]. Kennedia nigriscans an endophytic isolate which biosynthesized metabolites with curing significance on wounds and skin infections, so also NRRL 30562 (streptomyces species) was seen with an extreme antimicrobial activity against several infectious agents due to munumbicins A-D and munumbicins E-4 and E-5 it biosynthesized as an antibiotic, antibacterial peptide, actinomycin X2 and actinomycin D [11]. High diversified endophytic bacteria were isolated from medicinal plants of different area in Iran using hot method with evidence of producing antimicrobial activity, especially the endophytic actinobacteria that showed greater advantage of producing newly biological active compounds with therapeutical solution to pathogenic bacteria resistant of the prevailing antibiotics [12]. Furthermore, a report by Aljuraifani et al., [13] confirmed a high inhibitory activity of endophytic bacteria isolated from Moringa peregrine against both gram-positive and gram-negative. In the same year, Wang et al., [14] and his team reported diversified culture of bacterial endophytes from Dendrobium with high potential antimicrobial activity in diverse way, been the bacterial culture-independent. More so, the strains of wheat seeds Bacillus, Pseudomonas and Burkholderia were found to have great antimicrobial activity upon virulent bacteria [15].
Antioxidants are compounds that inhibits or detain the oxidation of oleaginous or other particles by obstructing the initiation or multiplication of oxidising serial reactions. The interest in knowing antioxidants sourced from plants has to be considered so as to improve and maintain human health, shelf-life prolonging, as well as retaining the quality of foods that are lipid-containing [16]. Plants have the capability of producing these important metabolites abundantly in form of phenolic compounds (phenolic acid and flavonoids), nitrogen compounds (amino acids, amines, alkaloids and chlorophyll derivatives), ascorbic acids and carotenoids [17]. The phenolic compounds have redox properties and its said to be the reason for the antioxidant activity, they play an important role by neutralizing and absorbing free radicals, extinguishing singlet and triplet oxygen or eroding peroxides [18]. Endophytic bacteria good source of antioxidant metabolites [19]. Endophytic bacteria isolated from Aloe vera produced potent antioxidants metabolites reaffirming their potency [20]. Many reports have proven that endophytes can produced useful antioxidants [21]. This study is aimed at verifying the potent of the bacterial endophytes from different parts of three selected medicinal plants for their antioxidant and antimicrobial cruciality. Generally, reports on bacterial endophytes are less compared to the fungal endophytes.
Collection of plant materials
Psidium guajava, Mangifera indica, Cassia occidentalis, Calotropis procera and Hibiscus rosa-sinensa healthy parts, namely to be leaves, roots, stem, bark and flower were selected and randomly collected at different location from Parul Institute of Applied Science’s biological/medicinal garden. The samples collected were kept separately in clean plastics and quickly brought to the laboratory to the maintain the freshness which were immediately used for the experimental work aimed.
Purification procedure and surface sterilization
Sequential sterilization technique was applied to achieve a successful isolation of the bacterial strains. Initially, freshly collected barks, flower, leaves, roots and stems are washed under tap water for 10-15 minutes and then in Tween 20 (a drop in 200 mL Shoot Dry Weight (SDW)) for 1 minute. The plant tissues were transferred into laminar air flow cabinet, rinsed three times with SDW. Sterilizing agents used were ethanol 70%-95% for 30 seconds to 4 minutes, hydrogen peroxide 0.05%-2%, chloride 0.02% to 0.2% for 30 seconds and sodium hypochlorite 1% to 5% for 2 to 10 minutes. All of which were used differently in the treatment of the explants.
Media used
Medium is what determines the type and number of endophytic microorganisms to be isolated from different plant tissue, so the choice of the medium is very important. The media that goes well with bacterial endophytes isolation is Nutrient Agar (NA). Nutrient agar was prepared and poured in petri plates for isolation of bacterial endophytes.
Isolation of bacterial endophytes
Following the final rinsing of the sterilized plant tissues surfaces in the laminar airflow, aseptically the surface of the stems was removed using a sterile scalpel, leaves were cut into pieces, so also the flower and the roots. The pieces were dried properly after which the pieces were implanted upon the nutrient agar plate. Each plate was independently inoculated with 2-3 small pieces of the plant organ and were incubated at 37°C to redeem the possible bacterial endophytes colonies maximally. The plates were observed between the period of 24 hrs to 48 hrs. Morphologically, the colonies of the different bacteria observed were sub-cultured and by streaking many times on nutrient agar plates to attain pure bacterial isolates. Lastly, the clarified endophytes were kept at 4°C for use.
Characterization and identification of bacterial endophytes
The strains of the bacteria isolated were experimentally identified by morphological characterization, microscopically by gram staining and biochemical tests including Indole test, Methyl Red test, Voges-Proskauer test, Citrate test (IMViC), Triple sugar iron test, oxidase test, catalase test, etc., for identification of the isolates [22].
Antimicrobial activity
The antimicrobial activity of the endophytes isolated was determined growing the culture overnight followed by centrifugation at 10,000 rpm for 4 minutes at 40°C and by pipetting 20 μl supernatant in the wells punched in Mueller Hinton Agar (MHA) plates with pre-cultured Escherichia coli and Staphylococcus aureus spread all over the surface of the agar plates using cotton swab [23].
Determination of antioxidant activity using the 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) radical scavenging method
The antioxidant activity of the plant extracts against DPPH was determined using the method proposed by Brand-Williams et al., [24]. Tracing the antioxidant activities of compounds is assigned to several mechanisms, like avoidance of chain initiation attaching with transition metalion catalysts, peroxides decomposition, reducing ability, radical scavenging capacity, prohibition of unseizable hydrogen abstraction [25]. A methanolic dilution of DPPH 1 × 10-4 M was prepared. Aliquots of 1 mL of each sample in the methanolic extract were collected (at 4 different concentrations such as 0.1, 0.5, 1 and 2 mg/mL; two replicates per sample and concentration) and had 2 mL of methanolic dilution of DPPH added. The mix was kept in the dark at room temperature for 16 min and absorbance was measured at 517 nm. The control was prepared with the methanolic dilution of DPPH. The results were expressed in milligram equivalents of quercetin per milligram of dry weight. The calibration line was established using the following concentrations of quercetin such as 0.001, 0.002, 0.005, 0.01, 0.02 and 0.04 mg/mL. The inhibition percentage/antioxidant activity was calculated using the formular mentioned below.
Inhibition (%)=((Absorbance of control-Absorbance of sample)/(Absorbance of control)) × 100
Plant material collection
The plant tissues of the five medicinal plants used in this work were collected from biological/medicinal garden of and were authenticated at department of Botany, Parul Institute of Applied Sciences.
Purification of endophytic bacterial culture
The plant tissues were independently purified by using different combination of 75% ethanol and 4% sodium hypochlorite in sterilising Psidium guajava, Cassia occidentalis, Calotropis procera and Hibiscus rosa-sinensa, while Mangifera indica was sterilized using 75% ethanol, 4% sodium hypochlorite and 0.1% mercuric chloride followed by rinsing with sterilized distilled water three times. After a successful surface sterilization, numerous endophytic bacterial colonies were recovered, series of streaking of the colonies was made on separate petri plates to attain a pure culture of each isolate. Figures 1A and 1B shows the pure of CPF-1 E. aerogenis and HBL-1 Achromobacter xylosoxidans (Figures 1A and 1B).
Figure 1: Pure culture of endophytic bacteria, (A): CPF-1 E. aerogenis; (B): HBL-1 Achromobacter xylosoxidans.
Characterization of endophytic bacteria
Thirty endophytic bacteria were isolated, they showed variability in both their morphological and physiological features. There are white, yellow, off white, maroon and brown-like/reddish colonies. Their spreading on the agar plates varied also some with clump, zigzag, feather-like, lightly/widely spread etc., 19 are gram-positive and 11 are gram-negative. Figures 2A and 2B shows microscopic view of CPF-1 E. aerogenis and HBL-1 Achromobacter (Figures 2A and 2B).
Figure 2: Microscopic view of endophytic bacteria, (A): CPF-1 E. aerogenis; (B): HBL-1 Achromobacter xylosoxidans.
Biochemical test
The results showed both positive and negative of Indole test, Methyl red test, Voges prokauer test and Citrate test. 26 are catalase producers, 25 are oxidase producers, 5 are lactose fermenters while 6 are not. 18 are seen with yellow slant and 12 with red slant, while 27 are seen with yellow butt and 3 are seen with red butt, whereas none of the endophytic bacteria indicate the presence of H2, but 3 produced gas. Identified endophytic bacteria showed 17 are Bacillus species, 4 Pseudomonas species, 2 Achromobacter, 2 Enterobacter, 1 Coccobacilli, 1 Klebsiella, 1 Siccibacter, 1 Citrobacter and 1 Escherichia (Tables 1 and 2).
| Gram positive | Gram negative |
|---|---|
| GL2 | GL1 |
| GL3 | GB2 |
| GL4 | GST1 |
| GL5 | COL3 |
| GB1 | COB1 |
| GB3 | COR1 |
| ML1 | CPL1 |
| ML2 | CPF2 |
| MB1 | CPS2 |
| MB2 | HBL3 |
| MB3 | HBST1 |
| COL1 | - |
| COL2 | - |
| COB2 | - |
| COR2 | - |
| CPF1 | - |
| CPS1 | - |
| HBL1 | - |
| HBL3 | - |
| 19 (63%) | 11 (36%) |
Note: GL: Psidium guajava Leaf; GB: Psidium guajava Bark; ML: Mangifera indica Leaf; MB: Mangifera indica Bark; COL: Cassia occidentalis Leaf; COB: Cassia occidentalis Bark; COR: Cassia occidentalis Root; CPF: Calotropis procera Flower; CPS: Calotropis procera Stem; HBL: Hibiscus rosa-sinensa Leaf.
Table 1: Gram staining of the total isolates.
| Isolates | Ind test | M-R test | V-P test | Ci test | Ca test | Oxi test | Lac test | Tripple sugar iron test | Identified species | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slant | Butt | H2S | Gas | |||||||||
| GL1 | - | - | - | - | + | + | NLF | Y | Y | - | - | Achromobacter xylosoxidase |
| GL2 | - | + | + | - | + | - | GP | R | R | - | - | Bacillusspecies |
| GL3 | - | + | - | - | + | + | GP | Y | Y | - | - | Bacillus megaterium |
| GL4 | - | + | + | - | + | + | GP | Y | R | - | - | Bacillus cereus |
| GL5 | - | + | + | - | + | - | GP | R | Y | - | - | Bacillus pacificus |
| GB1 | - | + | + | + | - | + | GP | Y | Y | - | - | Bacillus cereus |
| GB2 | - | + | + | + | + | + | LF | R | Y | - | - | Pseudomonas chlororaphis |
| GB3 | - | - | - | - | - | + | GP | Y | Y | - | + | Bacillus amyloliquefaciens |
| GST1 | - | + | - | - | + | + | NLF | Y | Y | - | + | Citrobacter koseri |
| ML1 | - | + | - | - | + | - | GP | R | Y | - | - | Bacillusspecies |
| ML2 | - | + | + | - | + | + | GP | R | Y | - | - | Bacillus amyloliquefaciens |
| MB1 | - | - | - | - | + | + | GP | Y | Y | - | - | Bacillus mojavensis |
| MB2 | - | + | + | - | + | + | GP | R | Y | - | - | Bacillus subtilis |
| MB3 | - | + | + | + | + | + | GP | R | Y | - | - | Bacillus pumilus |
| COL1 | - | + | - | - | + | + | GP | Y | Y | - | - | Bacillus amyloliquefaciens |
| COL2 | - | + | + | - | + | + | GP | R | Y | - | - | Bacillus pumilus |
| COL3 | - | + | + | + | + | - | LF | R | R | - | - | Klebsiella terrigina |
| COB1 | - | + | - | - | + | + | LF | Y | Y | - | - | Siccibacter colletis |
| COB2 | - | + | + | - | + | - | GP | R | Y | - | - | Bacillus anthraciis |
| COR1 | + | + | + | - | + | + | NLF | R | Y | - | - | Pseudomonas putida |
| COR2 | - | + | + | - | + | + | GP | Y | Y | - | - | Bacillus cereus |
| CPL1 | - | + | - | + | + | + | NLF | Y | Y | - | - | Pseudomonas gramnia |
| CPF1 | - | - | + | + | + | + | GP | R | Y | - | - | E. aerogenes |
| CPF2 | + | + | + | - | - | + | GP | Y | Y | - | + | Escherichia coli |
| CPS1 | - | + | + | - | + | + | GP | Y | Y | - | - | Bacillus oleronius |
| CPS2 | - | + | - | - | + | + | NLF | Y | Y | - | - | Pseudomonas chlororaphis |
| HBL1 | - | - | - | - | + | + | GP | Y | Y | - | - | Achromobacter xylosoxidase |
| HBL2 | - | + | + | + | + | + | GP | Y | Y | - | - | Bacillus pumilus |
| HBL3 | - | + | - | + | - | + | NLF | Y | Y | - | - | Coccobacilli |
| HBST1 | - | + | + | + | + | + | LF | Y | Y | - | - | Enterobacterspecies |
Note: GL: Psidium guajava Leaf; GB: Psidium guajava Bark; GST: Psidium guajava Stem; ML: Mangifera indica Leaf; MB: Mangifera indica Bark; COL: Cassia occidentalis Leaf; COB: Cassia occidentalis Bark; COR: Cassia occidentalis Root; CPL: Calotropis procera Leaf; CPF: Calotropis procera Flower; CPS: Calotropis procera Stem; HBL: Hibiscus rosa-sinensa Leaf; HBST: Calotropis procera Stem; IND: Indole; M-R: Methyl Red; V-P: Voges Prokaeur; Ci: Citrate; Ca: Catalase; Oxi: Oxidase; Lac: Lactose; NLF: Non Lactose Fermenters; LF: Lactose Fermenters; GP: Gram Positive; Y: Yellow; R: Red.
Table 2: Biochemical test result of the isolates and their identifications.
Antimicrobial activity
The antimicrobial activity was checked by using sample strains against Escherichia coli and Staphylococcus aureus. Their effectivity was verified through a method known as well diffusion and the comparison of the of the results with positive and negative controls affirm their potency or inactivity. Achromobacter xylosoxidans (GL-1), Bacillus megaterium (GB-1), Pseudomonas chlororaphis (CPS-2), Citro bacterkoseri (GST-1), Siccibacter colletis (COB-1) and Coccobacilli (HBL-3) showed resistance against Escherichia coli while the antimicrobial activity was noticed on Bacillus amyloliquefaciens GB-3 (12 mm) and E. aerogenes CPF-1 (>40 mm) isolates. There was a remarkable antioxidant activity when the isolates were tested against Staphylococcus aureus, only Bacillus amyloliquefaciens (GB-3) showed resistance but the remaining isolates were revealed to have antimicrobial activity, Achromobacter xylosoxidans (GL-1), Bacillus megaterium (GB-1), Pseudomonas chlororaphis (CPS-2), Citrobacter koseri (GST-1), Siccibacter colletis (COB-1), E. aerogenes (CPF-1) and Coccobacilli (HBL-3). Figures 3A and 3B shows antimicrobial activity of E. aerogenes CPF-1 and Coccobacilli HBL-3 against Escherichia coli and Staphylococcus aureus (Figures 3A and 3B and Table 3).
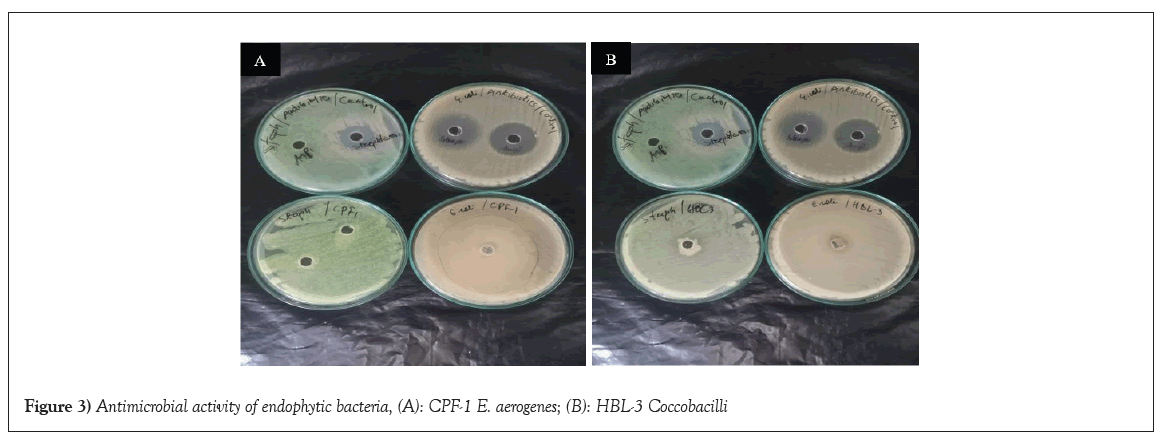
Figure 3: Antimicrobial activity of endophytic bacteria, (A): CPF-1 E. aerogenes; (B): HBL-3 Coccobacilli.
| Strains | Escherichia coli | Staphylococcus aureus |
|---|---|---|
| Achromobacter xylosoxidans (GL-1) | Red | 17 mm |
| Bacillus megaterium (GB-1) | Red | 20 mm |
| Pseudomonas chlororaphis (CPS-2) | Red | 18 mm |
| Bacillus amyloliquefaciens (GB-3) | 12 mm | Red |
| Citro bacterkoseri (GST-1) | Red | 13 mm |
| Siccibacter colletis (COB-1) | Red | 34 mm |
| E. aerogenes (CPF-1) | >40 mm | 16 mm |
| Coccobacilli (HBL-3) | Red | 15 mm |
Table 3: Antimicrobial activity of endophytic bacteria.
Antioxidant capacity
Overnight culture of the selected strains after centrifugation were the sample used to examine the antioxidant activity employing method of DPPH. Percentage of inhibition of the samples indicates its potent antioxidant activity. All the isolates showed appreciable percentage of inhibition which define them as good source of phenolic compound (inhibition percentage is depends on the concentration of the phenolic compound). Bacillus megaterium (GB-1) showed highest percentage of inhibition 93.72, followed by Bacillus amyloliquefaciens (GB-3) 93.50 and Siccibacter colletis (COB-1) 93.18. The percentage descends continuously with Citrobacter koseri (GST-1) 92.76, Achromobacter xylosoxidans (GL-2) 92.55, Coccobacilli (HBL-3) 92.01, Pseudomonas chlororapis (CPS-2) 90.84 and E. aerogenes (CPF-1) was expressed to have lowest percentage of inhibition as 90.40 (Figure 4).
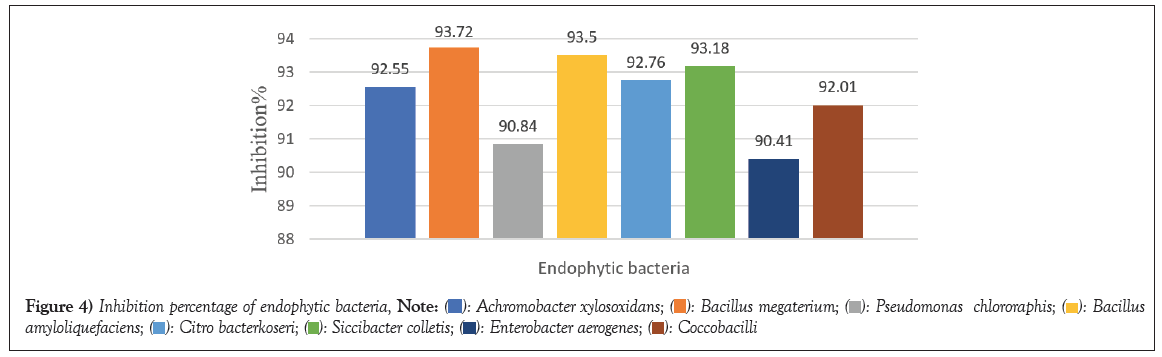
Figure 4: Inhibition percentage of endophytic bacteria, 
 .
.
The antimicrobial activity of endophytic bacteria isolated from plant tissues of four medicinal plants against pathogenic (gram positive and gram negative) bacteria was investigated. The superior of antimicrobial activity of the endophytic bacteria was observed against Staphylococcus aureus (gram positive) to that against Escherichia coli (gram negative). This investigation clearly revealed the possibility of using these endophytic bacteria as dependable antimicrobial agents. Our finding tallies with the view of Joo et al., [10] that antimicrobial metabolites are among the predominant compounds biosynthesize by endophytes. Egamberdieva et al., [26] also reported metabolites from endophytic bacteria which are proven to be excellent agents for therapeutic activity in providing relief, elimination of damaged cells and complete eradication of pathogens. Advantageously, endophytes could be an alternative in producing secondary metabolites known to be produced by their plant host as they both produced similar bioactive compounds, as it was reported Psidiium guajavaas as an antimicrobial, Mangifera indica as an antioxidant, Cassia occidentalisas an anticancer, Calotropis procera as an anti-inflammatory and Hibiscus rosa-sinensaas an antibiotic [27-31]. This could be reason for the isolated endophytic bacteria to synthesize potent metabolites with antimicrobial activity.
Many previous reports have shown the higher antimicrobial activity of the endophytic bacteria against gram positive than against gram-negative, which is also in line with our finding, we revealed that gram negative bacteria are more resistance to endophytic metabolites than gram positive [32]. In this report Bacillus amyloliquefaciens (GB-3) is the only isolate that showed resistance against gram positive bacteria (Staphylococcus aureus) but Achromobacter xylosoxidans (GL-1), Bacillus megaterium (GB-1), Pseudomonas chlororaphis (CPS-2), Citro bacterkoseri (GST-1), Siccibacter colletis (COB-1), Coccobacilli (HBL-3) and Enterobacter aerogenes (CPF-1) all showed antimicrobial activity. Contrarily, almost all the endophytic bacteria which are Achromobacter xylosoxidans (GL-1), Bacillus megaterium (GB-1), Pseudomonas chlororaphis (CPS-2), Citro bacterkoseri (GST-1), Siccibacter colletis (COB-1), Coccobacilli (HBL-3) showed resistance against gram negative except Bacillus amyloliquefaciens (GB-3) and Enterobacter aerogenes (CPF-1). Yusof et al., [33] reported more resistance in gram negative ZnO nanoparticles than gram positive.
Moreso, our finding also revealed the potency of metabolites of endophytic bacteria isolated from the back and stem of Psidium guajava, flower of Calotropis procera and bark of Cassia occidentalis for the first time. Reports related on endophytic bacteria related to this are very few, most of which are on fungal endophytes. Sahu et al., [34] expressed the antibiotic activities of endophytic bacteria from the root and leaves of Psidium guajava. Similarly, endophytic bacteria isolated from the root, leave and stem of Calotropis procera was reported to also have antimicrobial activity by Hamayun et al., [35]. Also, Kumar et al., [36] reported antimicrobial activity of endophytic bacteria from the root of Cassia occidentalis. To our knowledge all the reports mentioned here if not the only reports they are among the very few revealed findings concerning the antimicrobial activity of endophytic bacteria from Psidium guajava, Calotropis procera and Cassia occidentalis medicinal plants.
Antioxidant activity
The antioxidant activity of Achromobacter xylosoxidans (GL-1), Bacillus megaterium (GB-1), Pseudomonas chlororaphis (CPS-2), Citro bacterkoseri (GST-1), Siccibacter colletis (COB-1), Coccobacilli (HBL-3), Bacillus amyloliquefaciens (GB-3) and Enterobacter aerogenes (CPF-1) was studied by DPPH radical scavenging method. The entire endophytic bacteria tested showed admirable antioxidant potentiality. Hulikere et al., [37] conclude that phenolic compounds as the reason for antioxidants activity. We revealed that all the tested endophytic from the bark and stem of Psidium guajava, flower of Calotropis procera and bark of Cassia occidentalis in this work are good producers of phenolic compounds. The inhibition reported in the presence of this study may be as a result of the phenolic compounds.
Comparing the potency of the antimicrobial and antioxidant activity of the endophytic bacteria investigated in this work. It was noticed that the entire strains are more advantageous as good antioxidants metabolites producers than antimicrobial compounds producers. Nevertheless, both are important being the impact of the metabolites very active or not considering challengeable antimicrobial resistance and pathogenic infection account for high death as well as prolonged suffering in humans worldwide. Thus, efforts have been made untiredly, so also likeable attention needed has continuously been given to the creation of specific agents for a possible elimination, curing, providing relief and replacement of damaged/unwanted cells/tissues in the body. We believe this finding would contribute in providing safety in humans as well as healthy environment.
Endophytic bacteria revealed a clarity of being promising source of novel bioactive compounds conveying both antioxidant and antimicrobial activities. The isolates used in this work represents naturally significant machinery that could cheaply be used in producing bioactive metabolites flexible in designing several desired drugs. They were expressed to be mostly Bacillus Sp., Pseudomonas Sp., Achromobacter Sp., Enterobacter Sp., Siccibacter Sp., Coccobacilli Sp., Klebsiella Sp., Escherichia Sp. and Siccibacter Sp. as endophytic communities of Psidiium guajava, Mangifera indica, Cassia occidentalis, Calotropis procera and Hibiscus rosa-sinensa. Antioxidant screening of these bacterial isolates show high enzymatic activities, which have much Industrial value. Antimicrobial screening also indicates good zone of Inhibition. Those antimicrobial bioactive compounds will replace the severe use of antibiotics Therefore, it could be concluded that plant based endophytes are good cheap producers of notable secondary metabolites, for Pharma industry or Drug Industry this makes our study is attractive and important in present scenario.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Muhtari K, Sailaja I, She hawat BK, et al. Antimicrobial and antioxidant activities of endophytic bacteria associated with medicinal plants. AGBIR.2024;40(4):1178-1184.
Received: 11-Jun-2024, Manuscript No. AGBIR-24-138741; , Pre QC No. AGBIR-24-138741 (PQ); Editor assigned: 14-Jun-2024, Pre QC No. AGBIR-24-138741 (PQ); Reviewed: 28-Jun-2024, QC No. AGBIR-24-138741; Revised: 05-Jul-2024, Manuscript No. AGBIR-24-138741 (R); Published: 12-Jul-2024, DOI: 10.35248/0970-1907.24.40.1178-1184
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
