Agricultural and Biological Research
RNI # 24/103/2012-R1
Research Article - (2023) Volume 39, Issue 5
Sphaeranthus suaveolens (Forssk.) DC. and Argemone mexicana L. plant species are agricultural weeds that threaten crops and native plant diversity in East Africa, particularly in Tanzania. Yet, there have been few studies to assess whether their allelopathic effects inhibit plant germination and growth. Experiments were carried out in petri dishes and pots to investigate the inhibition effects (here referred to as negative allelopathy) of S. suaveolens leaf (SsL) and A. mexicana leaf (AmL) crude extracts on Zea mays L germination and seedling growth. Zea mays seeds and seedlings were treated with different crude concentrations of SsL and AmL to investigate their inhibition potential. The results showed that SsL and AmL crude extract concentrations delayed and/or reduced Z. mays germination, particularly at higher concentrations (70% and 100%). At these higher concentrations, few seeds germinated compared to the number of seeds germinated at lower concentrations (0%, 25%, and 50%). Further, it was found that Z. mays growth vigour was negatively affected as evinced by lower stem and root lengths, diameters, and total fresh biomass at higher concentrations of SsL and AmL. Although this study demonstrates the allelopathic effect of S. suaveolens and A. mexicana on Z. mays germination and growth, it also recommends further laboratory and field research experiments to investigate their allelopathic effects on other crops and native plants. However, this study advises that the management and control of these weeds be taken into account as their inhibitory effects could subsequently lead to a loss of plant diversity and crop productivity.
Agroecosystem; Biodiversity; Crops; Invasive; Plant ecology; Weeds
Invasive weeds have been a major problem in the conservation and agricultural sectors worldwide [1,2]. Most of them and their associated problems considerably impact global economic growth [3-7]. Some countries spent millions of dollars annually on the monitoring and control efforts of these weeds [8,9]. Invasive weeds are defined here as indigenous or exotic plant species that have the potential to invade and inhabit natural and semi–natural systems (e.g., protected areas and agroecosystems) and subsequently lead to negative effects on biodiversity and the livelihoods of local people [6,10,11]. Invasive weeds threaten the functioning and services of ecosystems as they alter patterns of biodiversity and their interactions [12,13]. In their invaded habitats, they compete with other plant species for essential resources (for instance-water or moisture, nutrients, light, and space) needed for germination, growth, and development [14]. Moreover, some of the invasive weeds, particularly flowering species, can compete for insect-pollinators with crops or native plants [7,15,16]. The competition for pollination services as a result of the sharing of insect-pollinators between invasive weeds and crops or native plants can affect pollination of flowering crops or natives by reducing visits to co-flowering plants [12,17,18]. Reducing pollinator visitations on crops or native plants can negatively affect their fruits and/or seed sets, ultimately threatening plant diversity and crop production [5,12].
Furthermore, invasive weeds have been reported to host insect pests and diseases that can disrupt and displace beneficial animals (e.g., pollinators and decomposers) [12,19] and native plant species that are important in ecosystems [14]. Because of this, invasive weeds are able to significantly affect food security and economic growth of smallholder farmers, and alter ecological processes by changing native plant composition (declining species richness or population size) in invaded environments [8]. The competitive and suppressive abilities of invasive weeds are attributed to various traits, including a lack of natural enemies in their new habitats and allelopathic effects [13]. Most of them do not have natural enemies within their new environments, as they possess anti–herbivorous and anti-microbial properties that discourage natural enemies (i.e., insects and herbivores) from foraging on them [10,20,21]. Moreover, they have a large seed bank and the ability to resist extreme dryness and poor soil [22-24]. Also, they have various mechanisms, i.e., contaminated soils, seed products, and crops, that make their seeds spread easily [23,25]. This makes the invasive weeds dominant in the invaded habitats, subsequently outcompeting or suppressing co–occurring native plants or crops [14].
In addition, some invasive weeds have been suspected to possess negative allelopathic effects, which contribute to their invasion and suppressive success [10]. Allelopathy refers to the positive (favourable) or negative (adverse) effect of one plant species on another (neighbouring plant) due to the direct or indirect release of allelochemicals from live or dead plants. Invasive weeds’ secondary metabolites (or allelochemicals), which can be found in the stem, roots, leaves, flowers, and fruits, are responsible for allelopathic effects [1,14,21]. Negative allelopathy is the ability of a plant to suppress or prevent neighbouring native plants or crops from germination and growth by releasing allelochemicals [26-28]. Invasive weeds with negative allelopathic effects have been reported to suppress the germination, growth and development of adjacent plant species [26,29,30]. Also, some studies have asserted that such weeds have the ability to cause imbalances of nutrients and a loss of soil fertility and beneficial microbial community [1,14]. Because of this, most invasive weeds are able to negatively affect crop production if they invade agricultural fields [20,28,31]. Thus, it is becoming imperative to investigate the allelopathic effects of invasive weeds in our environment, control them, and look at the possibilities of utilizing their metabolites to suppress other deleterious invasive plants.
Sphaeranthus suaveolens (Forssk.) DC. (Asteraceae) and Argemone mexicana L. (Papaveraceae) are among the invasive weeds that have the capacity to colonize disturbed and undisturbed environments, e.g., agricultural fields, rangelands, and pasturelands in sub-Saharan African countries [22,25,26,29]. They threaten food production and biodiversity in these countries as they exhibit negative allelopathic effects that inhibit the germination and growth of crops and native plants [25,29,32]. In Tanzania, S. suaveolens and A. mexicana have been reported as agricultural weeds of maize (Zea mays L.), common bean (Phaseolus vulgaris L.), and rice (Oryza sativa L) [23,32]. As a result, they are suspected of threatening the integrity of agroecosystems and food production in the country [1,14,23,32]. Examples of phenolic allelochemicals found in S. suaveolens and A. mexicana that are responsible for allelopathic effects are shown in Figures 1a and 1b.
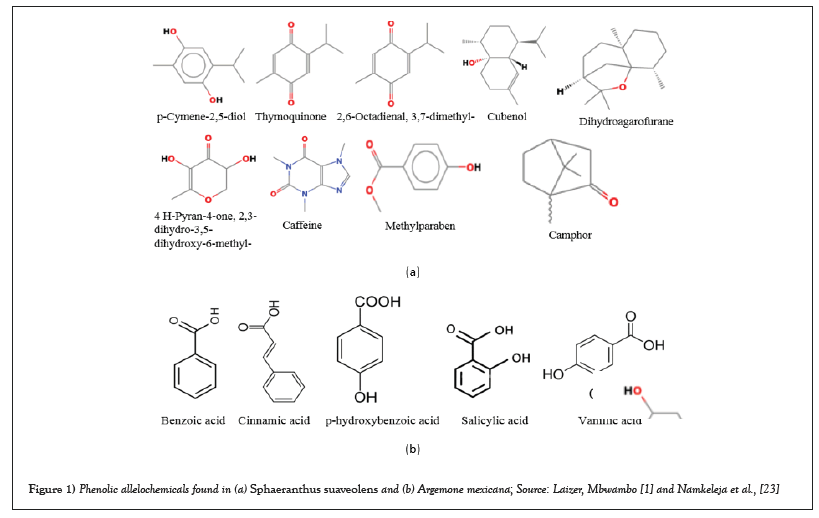
Figure 1: Phenolic allelochemicals found in (a) Sphaeranthus suaveolens and (b) Argemone mexicana; Source: Laizer, Mbwambo [1] and Namkeleja et al., [23].
Previous studies showed that the germination and seedling growth of tomato (Solanum lycopersicum), finger millet (Eleusine coracana), and cucumber (Cucumis sativus) were suppressed by A. mexicana leaf extract, while O. sativa and P. vulgaris were inhibited by S. suaveolens extracts [26,33-35]. Despite the allelopathic effects of S. suaveolens and A. mexicana being reported on various crops, there has been no study, particularly in Tanzania, carried out to investigate the allelopathic or suppressive effects of S. suaveolens and A. mexicana on the germination and growth of Z. mays. Having knowledge about these effects on Z. mays could increase the management and control efforts of the invasive weeds in order to increase crop production. Thus, the study was conducted in laboratory (petri dish experiments) and screen house (pot experiments) settings to investigate the allelopathic effects of S. suaveolens and A. mexicana leaf crude extracts on Z. mays germination and seedling growth. It was hypothesized that S. suaveolens and A. mexicana leaf crude extract concentrations would negatively affect seed germination and growth vigour of Z. mays.
Characteristics of study plants
Sphaeranthus suaveolens is an annual branched herbaceous plant that reproduces by seeds (Figures 2a and 2b). It has a taproot and a cylindrical stem with simple and elongated leaves [36]. Its pointed leaves measure up to 10 cm long and 4 cm wide [36]. It grows while spreading along the ground, and when it is upright or erect, S. suaveolens grows up to 60 cm tall [14]. Its globular or spherical inflorescences of violet or purple colour are a group or multitude of small tubular flowers with 1-2 cm in diameter (Figure 2a). Although its native range is Egypt to Tropical Africa, S. suaveolens is an invasive weed in Eritrea, Egypt, Kenya, Ethiopia, Tanzania, the Congo, and Uganda. It is commonly spread in pastures, swampy areas, cultivated farmlands, and irrigated crops. Sphaeranthus suaveolens heavy infestation has been linked with deleterious effects on the growth of native plants and a decline in crop yields. On the other hand, A. mexicana is an invasive herb (with prickles) that grows up to 1 m tall [22,37,38]. It has scentless yellow flowers whose diameter is 4-5 cm in Figure 2b, and spiny black spherical seeds of 5-11 cm long [25,26] and spiny obovate capsule of 3 cm long [22]. Although A. mexicana is native to Tropical America, it is an invasive weed in Botswana, Côte d'Ivoire, Cuba, Kansas, Mauritius, Syria, Tanzania, and Zimbabwe [25,26,32]. However, A. mexicana has also been naturalized in other countries, and it is thus considered an agricultural weed [24].
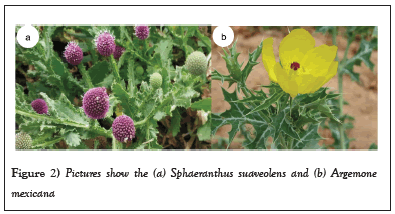
Figure 2: Pictures show the (a) Sphaeranthus suaveolens and (b) Argemone mexicana.
Several studies have revealed the health risks of A. mexicana to both humans and animals [22,38]. Being toxic and having prickles, A. mexicana is avoided by livestock [23,32] while S. suaveolens has been observed foraging by goats and sheep (personal observation). Its prickles also cause nuisances to farmers during farming and harvesting [22,25,39]. Despite having allelopathic effects, S. suaveolens has no prickles, and its health-related risk has not been studied compared to A. mexicana.
Preparation of S. suaveolens and A. mexicana leaf crude extract
Sphaeranthus suaveolens leaves (SsL) and A. mexicana leaves (AmL) were collected at Mbeya University of Science and Technology's (MUST) main campus (8° 56.24′ S and 33° 25.04′ E, 1636 m a.s.l.), MUST farms (8° 56.45′ S and 33° 25.40′ E, 1643 m a.s.l.), and Iyunga (8° 55.85′ S, 33° 25.05′ E, 1616 m a.s.l.) in the Mbeya region in May 2022. The leaves were collected early in the morning (between 6:00 a.m. and 7:00 a.m.) before sunrise to avoid degradation of allelochemicals by the sun [21]. Collected SsL and AmL samples were stored in plastic paper bags and transported to the MUST biology laboratory (8° 56.56′ S and 33° 25.21′ E, 1651 m a.s.l.). The leaves were cleaned thoroughly using water to remove any soil and/or debris. Later, the SsL and AmL samples were air dried at room temperature indoors (for 33 days) to avoid loss or degradation of allelochemicals by Ultraviolet (UV) light [21]. The dried SsL and AmL were separately ground into powder and stored in envelopes. About 100 g of SsL and AmL powder was measured using a digital balance and soaked in 1 l of distilled water. The crude was stored in a 4 l container for 48 h indoors, and afterwards, the crude was filtered using muslin cloth aqueous extract. To obtain different aqueous concentrations, i.e., 0%, 25%, 50%, 75%, and 100% (w/v) of SsL and AmL (100 ml each), relative to the original extract, the filtrates were diluted with distilled water [21,26]. The number of seeds germinated, seedling stem heights and diameters, root lengths, and fresh biomass were used as indicators of the allelopathic effects of SsL and AmL on Z. mays [35].
Germination experiment
The Z. mays seeds were purchased from Ikuti market (S8° 56.07′, E33° 25.18′, 1630 m) in Mbeya region, Tanzania, in May 2022. Before starting the germination experiment, undamaged seeds were sorted and air-dried for ten days. To investigate the allelopathic effect of SsL and AmL crude extracts on Z. mays seed germination, petri dish experiments were conducted in the biology laboratory at MUST (S8° 56.56′, E33° 25.21′, 1651 m). Five glass petri dishes (each with a 70.84 cm2 surface area) per treatment were used and then replicated five times to make 25 petri dishes for SsL and 25 for AmL treatments. Prior to sowing fifteen (15) Z. mays seeds, each petri dish was rinsed with clean water, dried, and lined with absorbent cotton wool. The seeds were irrigated ad libitum (i.e., kept moist) with different concentrations, i.e., 0%, 25%, 50%, 75%, and 100% (w/v) of SsL and AmL. The number of germinated seeds was recorded daily for 14 days. Petri dishes’ positions were randomized three times per week to ensure an equally distributed distribution of sunlight. In this experiment, the criteria used for seed germination were the emergence of the radicle [26]. The number of seeds germinated was calculated and compared between crude concentrations of SsL and AmL.
Seedling growth experiment
Growth experiments were conducted in a screen house at MUST (8° 56.61′ S and 33° 25.05′ E, 1646 m a.s.l.) to prevent insects, i.e., aphids and white flies, from damaging Z. mays seedlings. Fifteen seeds (15) of Z. mays were sown in fifty (50) pots (3 l) each, i.e., 25 pots for SsL and 25 for AmL treatments. At the time of sowing, pots were watered thoroughly with 0.5 l per pot. On the fifth day of germination, Z. mays seedlings were irrigated three times per week with different SsL and AmL crude concentrations of 0%, 25%, 50%, 75%, and 100% (w/v). In addition, the seedlings were sprayed ad libitum with SsL and AmL crude concentrations twice per week using a hand sprayer [21,26]. The irrigation and spraying of seedlings with SsL and AmL crude concentrations took 20 days between May and June 2022. Pot's positions were randomised three times per week to ensure an equally distributed distribution of sunlight. At the end of the experiment, seedling stem and root lengths, diameters, and total fresh biomass were measured to assess the allelopathic effects of SsL and AmL crude extracts on Z. mays growth vigour [14,21,32]. The Z. mays seedlings' total fresh biomass was determined using an analytical digital balance; the diameter (above the first two leaves) using a digital calliper; and the seedling stem and root lengths using a metre ruler.
Statistical data analysis
The number of seeds germinated and growth parameters (fresh biomass, stem and root lengths, and stem diameters) of Z. mays were compared for different SsL and AmL crude extract concentrations using a one-way ANOVA and Kruskal-Wallis for parametric and non-parametric data, respectively [14,21]. Levene’s test and Shapiro-Wilk test were used to test for equal variance and normality for all data, respectively. When the parametric assumptions were not confirmed after transformations (using either Box-Cox or log transformations), the non–parametric Kruskal-Wallis test was used. Significant differences were confirmed using the post hoc Tukey-Kramer HSD and Mann-Whitney pairwise comparison tests. A 0.05 significance level was used for all the tests. Statistical tests were performed with origin version 9.0 SR1 (2013).
Seed germination under treatments
The results show that SsL and AmL crude extracts possess negative allelopathic effects on Z. mays, which suppress its germination at higher concentrations (75% and 100%, Figure 3). The average number of Z. mays seeds germinated under these concentrations was reduced compared to those germinated at lower concentrations (25% and 50%) as well as control (0%). The result revealed further that Z. mays seed germination decreased with increasing SsL and AmL crude concentrations (Figure 3). For instance, the number of Z. mays seeds that germinated at 25% and 50% is about two times that of those that germinated at 75% and 100% (Figure 3). Overall, the number of seeds germinated at lower concentrations (i.e., 25% and 50%) and in the control differed significantly from those germinated at higher concentrations (SsL: H (4,20) =17.65, p=0.0019, F (4,20) =5.28, p=0.0046, AmL: F (4,20) =21.57, p<0.0001, Figure 3). In addition, it was found that high concentrations of SsL and AmL delayed the germination of Z. mays seeds compared to lower concentrations, which had a high germination rate in the early days (Figure 4). For instance, the seeds treated with SsL concentrations of 100% started to germinate on day 3 of the experiment (Figure 4).

Figure 3: Mean number of Z. mays seed (± SE) germinated under different concentrations of Sphaeranthus suaveolens (left) and Argemone mexicana (right) leaf crude extracts over a 14-day experiment in petri dishes. The germination of Z. mays seeds was reduced at high concentrations and decreased with increasing SsL and AmL concentrations.
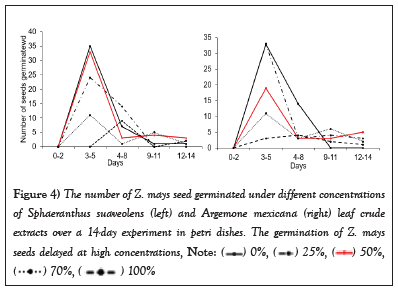
Figure 4: The number of Z. mays seed germinated under different concentrations of Sphaeranthus suaveolens (left) and Argemone mexicana (right) leaf crude extracts over a 14-day experiment in petri dishes. The germination of Z. mays seeds delayed at high concentrations, 
 .
.
Seedling growth parameters under treatments
Seedlings growth vigour in Z. mays was negatively affected by SsL and AmL crude extracts. The seedlings growth parameters (stem heights and diameters, root lengths, and total fresh biomass) under 75% and 100% SsL and AmL crude concentrations were significantly reduced (Table 1 and Figure 5). The growth parameters of Z. mays differed significantly across concentrations of SsL and AmL (Table 1). Figure 5 shows a decrease in stem and root lengths of Z. mays when treated with SsL and AmL crude extracts, particularly at high concentrations. Stem and root lengths (Mean ± SE) of Z. mays seedlings treated with SsL and AmL crude extracts differed significantly across different concentrations (Table 1). At 100% AmL concentrations, the stem length of Z. mays was reduced by 6.9 ± 0.0 cm, 5.8 ± 0.8 cm, and 3.8 ± 0.0 cm compared to 25%, 50%, and 0%, respectively (Figure 5 and Table 1). Similarly, the mean (± SE) stem and root lengths of Z. mays seedlings treated with 100% SsL crude concentrations were about six times shorter than those treated with lower concentrations (Table 1 and Figure 5). However, the mean (± SE) stem and root length of Z. mays seedlings was about two times shorter when treated with 100% of AmL crude extract concentrations (Table 1 and Figure 5).
| Growth parameters | Crude extract concentration (%) | Statistical value | ||||
|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 75 | 100 | ||
| Zea mays treated with SsL | ||||||
| Stem length | 7.7 ± 0.1a | 6.9 ± 0.1a | 5.9 ± 0.1b | 3.3 ± 0.1c | 1.0 ± 0.0d | H (4,20)=22.59, p=0.0002 |
| Root length | 7.9 ± 0.3a | 7.1 ± 0.1a | 5.0 ± 0.1b | 2.9 ± 0.1c | 1.2 ± 0.0d | H (4, 20)=22.60, p=0.0002 |
| Stem diameter | 3.7 ± 0.1a | 3.0 ± 0.0b | 2.2 ± 0.1c | 1.4 ± 0.1d | 1.2 ± 0.2d | F (4,20)=219.30, p<0.0001 |
| Fresh biomass | 2.8 ± 0.1a | 2.0 ± 0.1b | 1.7 ± 0.1c | 1.3 ± 0.1d | 1.1 ± 0.4d | F (4,20)=226.40, p<0.0001 |
| Zea mays treated with AmL | ||||||
| Stem length | 11.0 ± 01a | 9.9 ± 0.9b | 7.9 ± 0.0c | 5.9 ± 0.0d | 4.1 ± 0.1e | H (4,20)=23.08, p=0.0001 |
| Root length | 6.1 ± 0.1a | 5.6 ± 0.1b | 5.2 ± 0.0c | 4.1 ± 0.0d | 3.1 ± 0.1e | H (4,20)=22.39, p=0.0002 |
| Stem diameter | 4.9 ± 0.0a | 4.5 ± 0.1b | 4.0 ± 0.1c | 3.0 ± 0.1d | 2.2 ± 0.1e | F (4,20)=22.72, p=0.0001 |
| Fresh biomass | 1.1 ± 0.0a | 0.7 ± 0.2b | 0.7 ± 0.0b | 0.6 ± 0.0b | 0.3 ± 0.0c | F (4,20)=26.68, p<0.0001 |
Note: Values with different letter(s) in a row are significantly different by Tukey–Kramer HSD and Mann–Whitney pairwise comparison tests at p=0.05.
Table 1: Kruskal-Wallis rank sum and one-way ANOVA test of Z. mays seedling parameters after 20 days of S. suaveolens and A. mexicana leaf crude extract treatment in a screen house experiment.
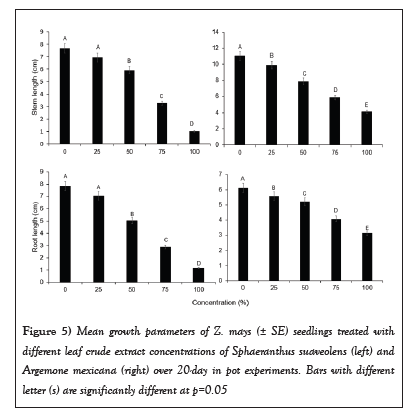
Figure 5: Mean growth parameters of Z. mays (± SE) seedlings treated with different leaf crude extract concentrations of Sphaeranthus suaveolens (left) and Argemone mexicana (right) over 20-day in pot experiments. Bars with different letter (s) are significantly different at p=0.05.
Furthermore, the stem diameter and total fresh biomass (Mean ± SE) of Z. mays seedlings treated with different crude concentrations of SsL and AmL differed significantly (Table 1 and Figure 6). The diameter of seedlings treated with 75% and 100% crude concentrations was slightly smaller than that of those treated with 0%, 25%, and 50% concentrations (Table 1 and Figure 6). For instance, the diameter at lower concentrations (25% and 50%) of SsL and AmL was about two times that at higher concentrations (75% and 100%). The total fresh biomass (Mean ± SE) of Z. mays seedlings was reduced at higher concentrations of SsL and AmL (Table 1 and Figure 6). The fresh biomass of Z. mays seedlings treated with 75% and 100% SsL concentrations was about two times smaller than the seedlings at lower concentrations (Table 1 and Figure 6).
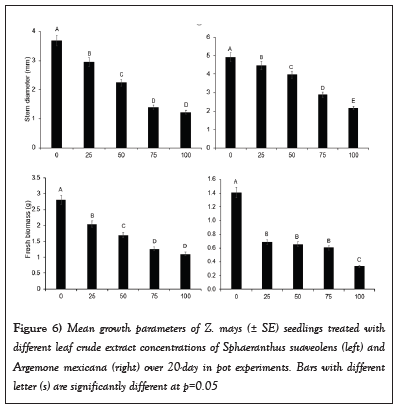
Figure 6: Mean growth parameters of Z. mays (± SE) seedlings treated with different leaf crude extract concentrations of Sphaeranthus suaveolens (left) and Argemone mexicana (right) over 20-day in pot experiments. Bars with different letter (s) are significantly different at p=0.05.
The study found that S. suaveolens and A. mexicana leaf crude extracts exhibit negative allelopathic effects on the germination and growth of plants. This is evidenced by the reduced seed germination and seedling growth of Z. mays. This result suggests further that S. suaveolens and A. mexicana's water-soluble phenolic allelochemicals have inhibitory effects on Z. mays. The germination and seedling growth of Z. mays were strongly suppressed, particularly at higher crude concentrations of SsL and AmL. This shows that the effectiveness of S. suaveolens and A. mexicana crude extracts is dosage–dependent, as supported by the preceding studies [21,40,41]. The S. suaveolens and A. mexicana crude extracts negatively affected Z. mays growth vigour, as evinced by lower stem and root lengths, stem diameter, and fresh biomass at high concentrations. This is also shown by the total number of Z. mays seeds that germinated at these concentrations.
Since S. suaveolens and A. mexicana affect fresh biomass, which is a vital factor for a plant to withstand physical stresses from the environment, they affect the ability of Z. mays to endure harsh environmental conditions [14].
Furthermore, reduced root length may have affected the uptake of nutrients and water, which are needed for plant physiological and metabolic activities to take place. This might be the reason for reduced growth, which is demonstrated by lower stem lengths in Z. mays. The observed lower stem and root lengths in this study indicate a negative effect on the survival and productivity of Z. mays. This is because most plants with shorter stem and root lengths fail to compete with other plants for water and minerals from the ground to ensure their survival [14]. These results are generally consistent with previous studies that found that S. suaveolens and A. mexicana inhibited the germination and growth of plants and crops [23,26,29,33,39,42].
For instance, Laizer et al., [43] established that at higher crude concentrations of S. suaveolens, germination and seedling growth of P. vulgaris and O. sativa were strongly inhibited. On the other hand, Salih et al., [26] reported a maximum inhibition of seed germination, stem and root lengths, and biomass of Corchorus olitorus L and Cassia senna L at high concentrations of A. mexicana. Further evidence that supports the current study reveals that crude extracts of A. mexicana suppressed the seed germination, shoot height, and root length of Brachiaria dictyoneura L and Clitoria ternatea L seedlings [23]. Thus, inhibition of seed germination and growth of Z. mays in the current study could be due to allelochemicals present in S. suaveolens and A. mexicana leaves. These allelochemicals might have interfered with Z. mays metabolic and physiological mechanisms that trigger seed germination and seedlings in the plant [21,44-46]. Other studies have also asserted that S. suaveolens and A. mexicana possess phenolic allelochemicals that are responsible for negative allelopathic effects on other plants [1,14,23].
For S. suaveolens, some examples of the allelochemicals reported from previous studies that might exhibit negative allelopathic effects include but are not limited to p-Cymene-2,5-diol, geranyl acetate, cubenol, methylparaben, dihydroagarofurane, guaiol and thymoquinone. Allelochemicals reported from A. mexicana to inhibit germination and growth of plants include but are not limited to benzoic acid, cinnamic acid, salicylic acid, vanillic acid and p-hydroxybenzoic acid [22,29,32]. Previous studies claim that A. mexicana uses p-hydroxybenzoic and vanillic acids to interfere with the water balance and physiological characteristics of adjacent plants, consequently inhibiting their growth [22,34]. As a result, the root activity is inhibited, and the amount of chlorophyll is reduced, eventually affecting the rate of photosynthesis [21,34]. Moreover, in their experiment, Barkosky and Einhellig [33] reported that p-hydroxybenzoic and vanillic acids were responsible for inhibition of germination and growth of soybean (Glycine max) and eggplant (Solanum melongena).
Additionally, flavonoids and terpenoids present in S. suaveolens have also been reported to inhibit the growth of plants [1]. This indicates that they might be responsible for reduced seed germination and growth of Z. mays. Besides, the ability of A. mexicana to suppress the germination and growth of Z. mays in this study could be due to salicylic and cinnamic acids [29]. These allelochemicals were previously reported to inhibit germination and seedling growth of cowpea (Vigna unguiculata) [42,47] and S. melongena [48] at high concentrations. Therefore, these allelochemicals that are present in S. suaveolens and A. mexicana might be responsible for the observed negative allelopathic effects on germination and growth of Z. mays.
However, it should be agreed that the effectiveness of allelochemicals present in an invasive plant can be species–specific. This means that some plant species may experience positive allelopathic effects while others may experience negative effects. For instance, according to the findings of Burhan and Shaukat [29], the effects of A. mexicana phytotoxin were species-specific, as not all plant species studied were equally inhibited by the extract.
In general, S. suaveolens and A. mexicana crude extracts have the ability to inhibit the germination as well as the early-growth of crops and other plants, as evinced from this study.
This is the first study to investigate the allelopathic effects of S. suaveolens on the germination and growth of Z. mays in East Africa. It is also the first to demonstrate the allelopathic effects of both S. suaveolens and A. mexicana on Z. mays germination and seedling growth in Tanzania. These effects are suspected to be caused by water-soluble allelochemicals present in S. suaveolens and A. mexicana in Z. mays. Since S. suaveolens and A. mexicana reveal negative allelopathic effects on plants, they should be controlled to prevent their effects on crop production. Nevertheless, working with the local communities, especially smallholder farmers and pastoralists, to combat and control S. suaveolens and A. mexicana is imperative because they are directly affected by these invasive weeds. Although this study reveals the allelopathic effects of S. suaveolens and A. mexicana leaf extract on Z. mays germination and growth, further field research is required to investigate these effects on other crops and native plant species.
The author thanks Elton O. Msuya, Athanas Martin, Belinda Wilson, Elias M. Justine, and Livini T. Aloyce for helping in setting up the experiments, collecting plant leaf samples, and recording the data. He also thanks two colleagues for their input and help in shaping and polishing the manuscript. Last but not least, the author is grateful to the management of Mbeya University of Science and Technology for in-kind support that includes laboratory and screen house equipment and space.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
Citation: Ojija F. Allelopathic effects of Sphaeranthus suaveolens (Forssk) DC and Argemone mexicana L leaf crude extract on Zea mays L germination and growth. AGBIR.2023;39(5):651-656.
Received: 17-Aug-2023, Manuscript No. AGBIR-23-111684; , Pre QC No. AGBIR-23-111684 (PQ); Editor assigned: 21-Aug-2023, Pre QC No. AGBIR-23-111684 (PQ); Reviewed: 04-Sep-2023, QC No. AGBIR-23-111684; Revised: 12-Sep-2023, Manuscript No. AGBIR-23-111684 (R); Published: 19-Sep-2023, DOI: 10.35248/0970-1907.23.39.651-656
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
